Clinical Trial Roadmap Slide for PPT Presentation

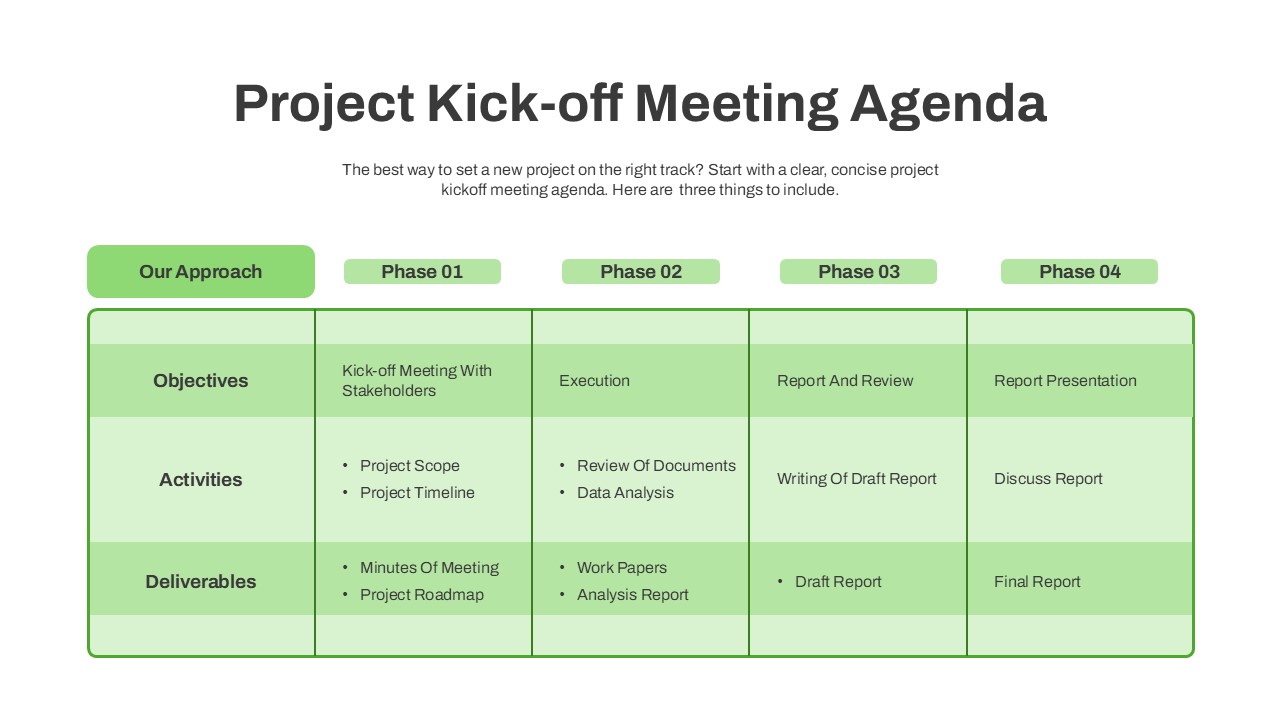
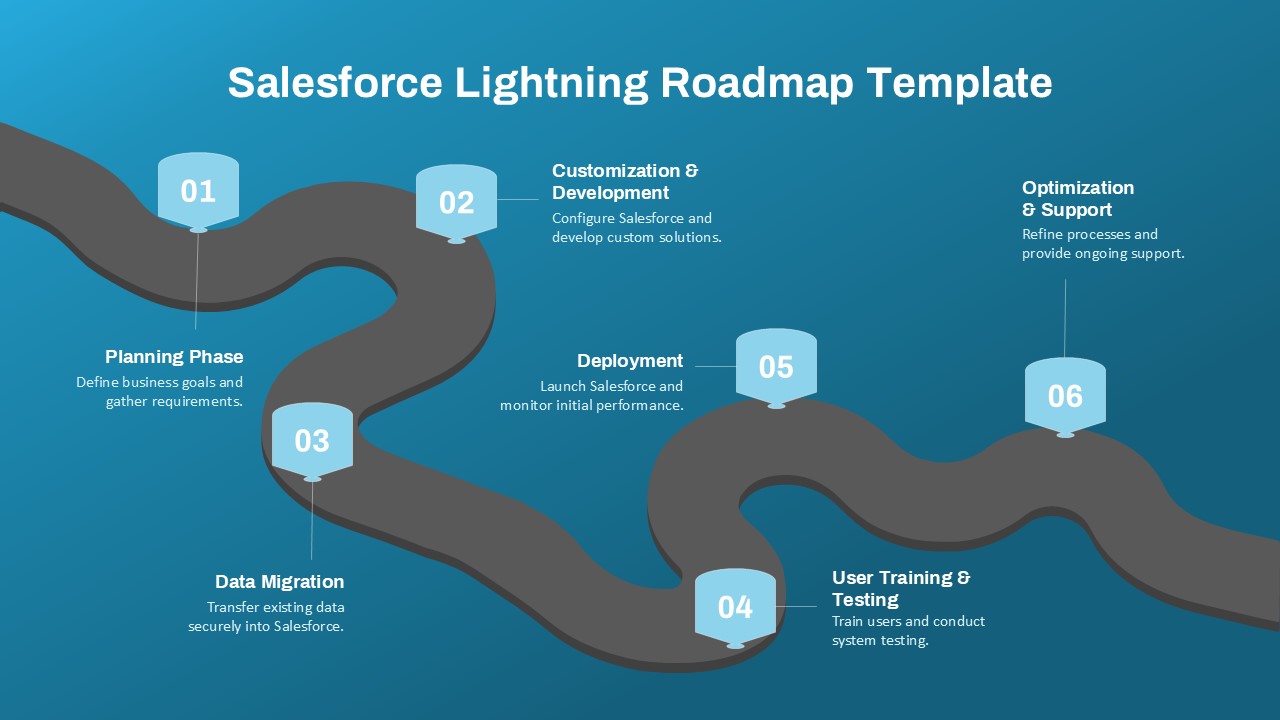
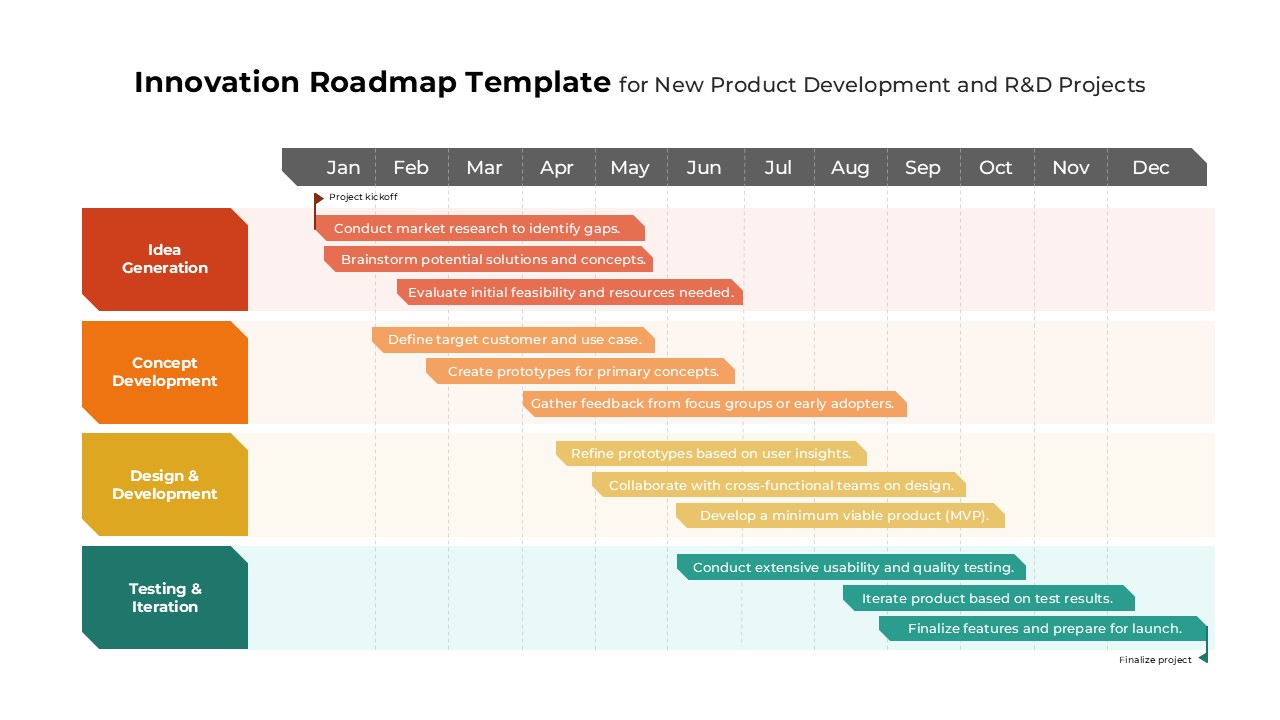
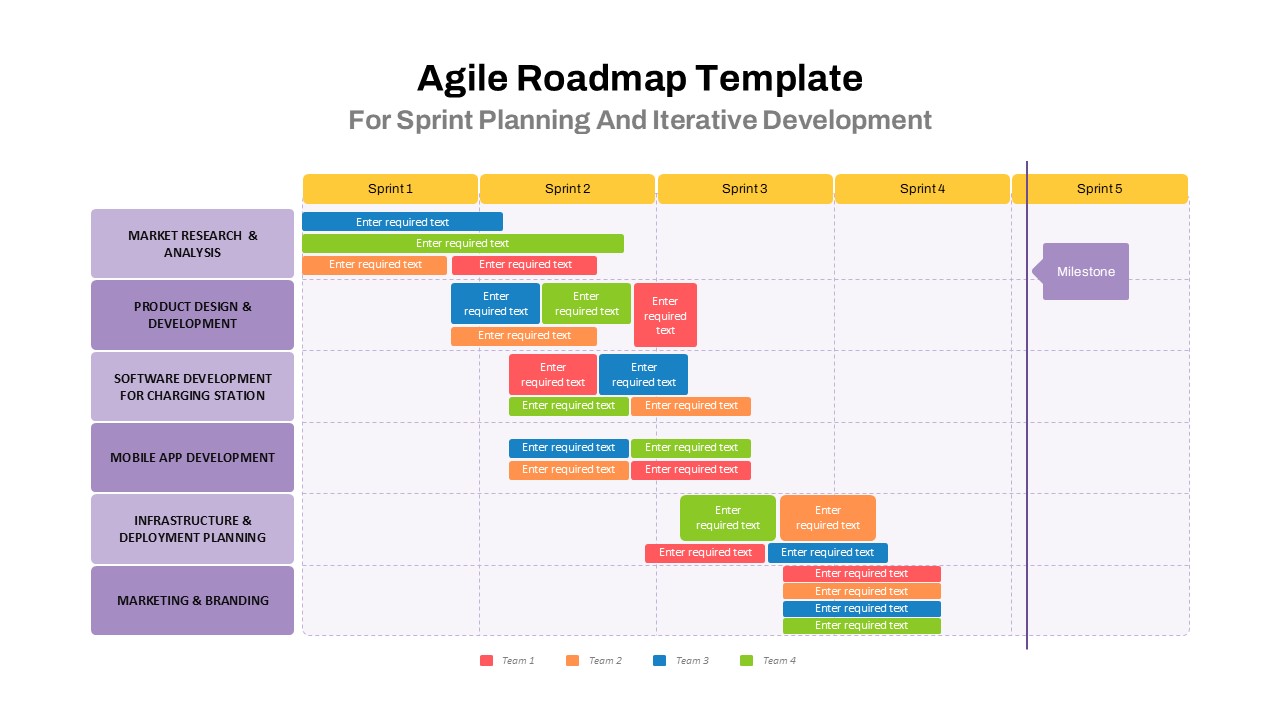
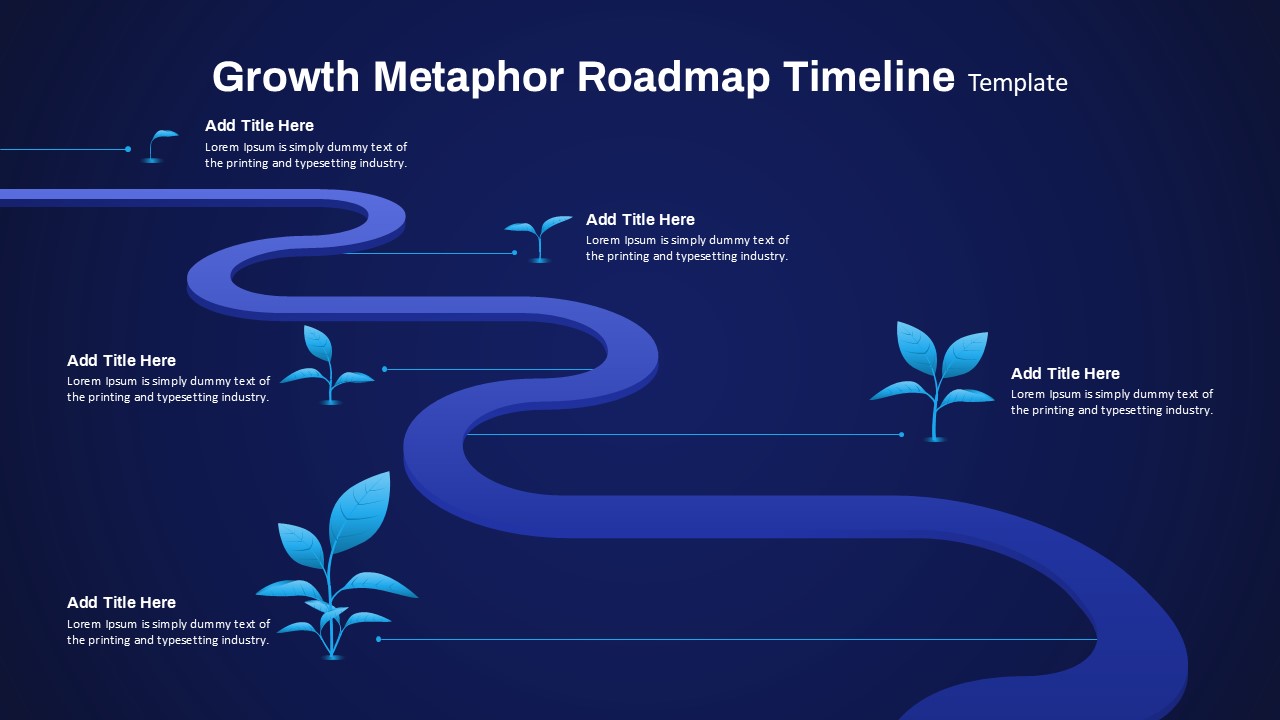
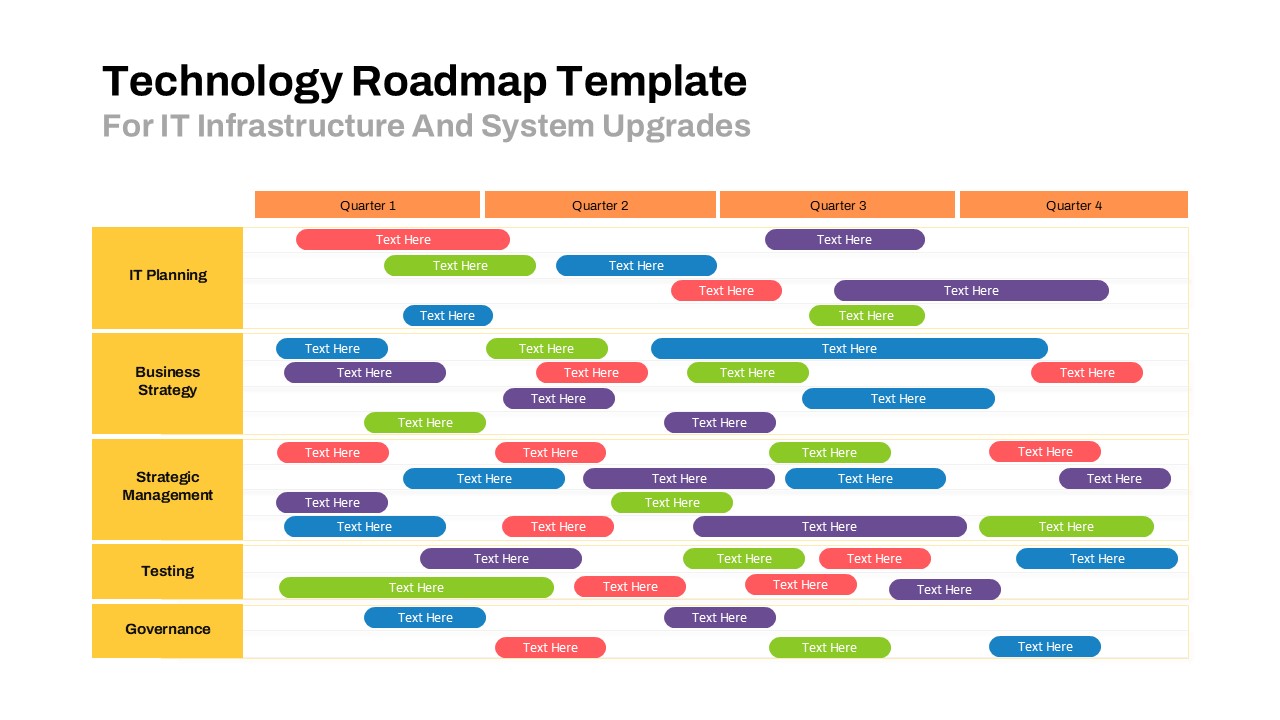
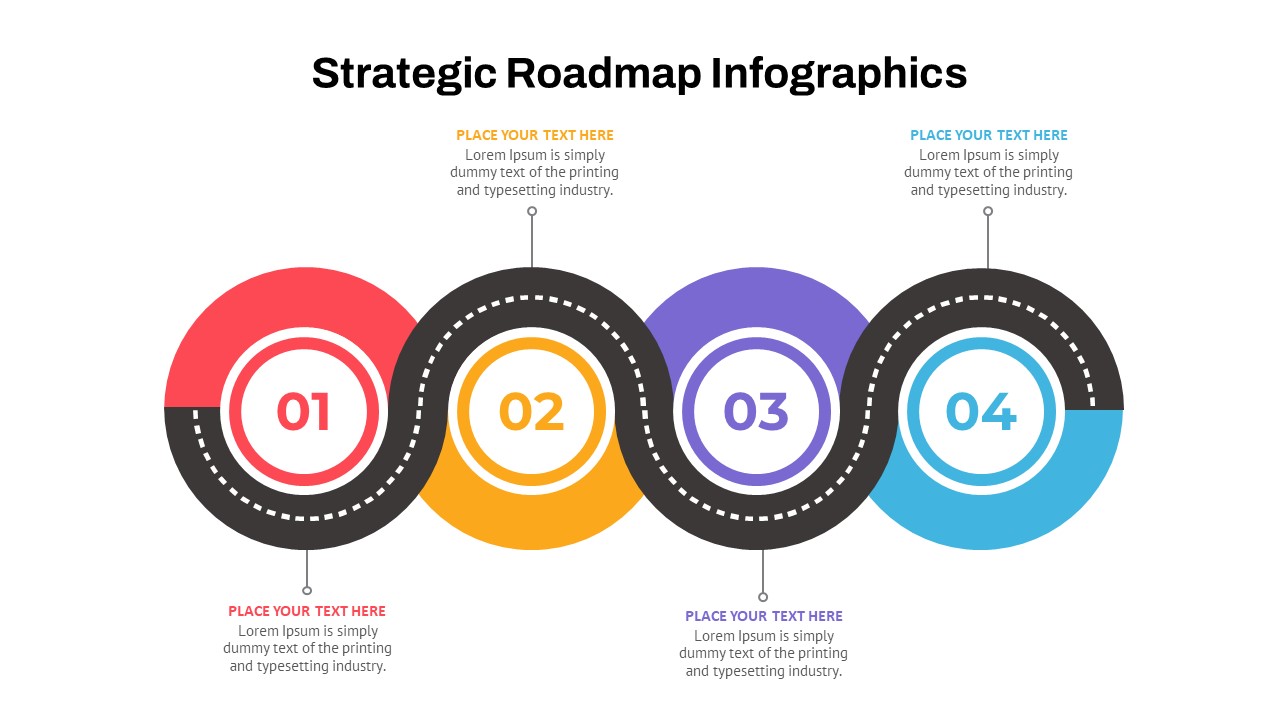
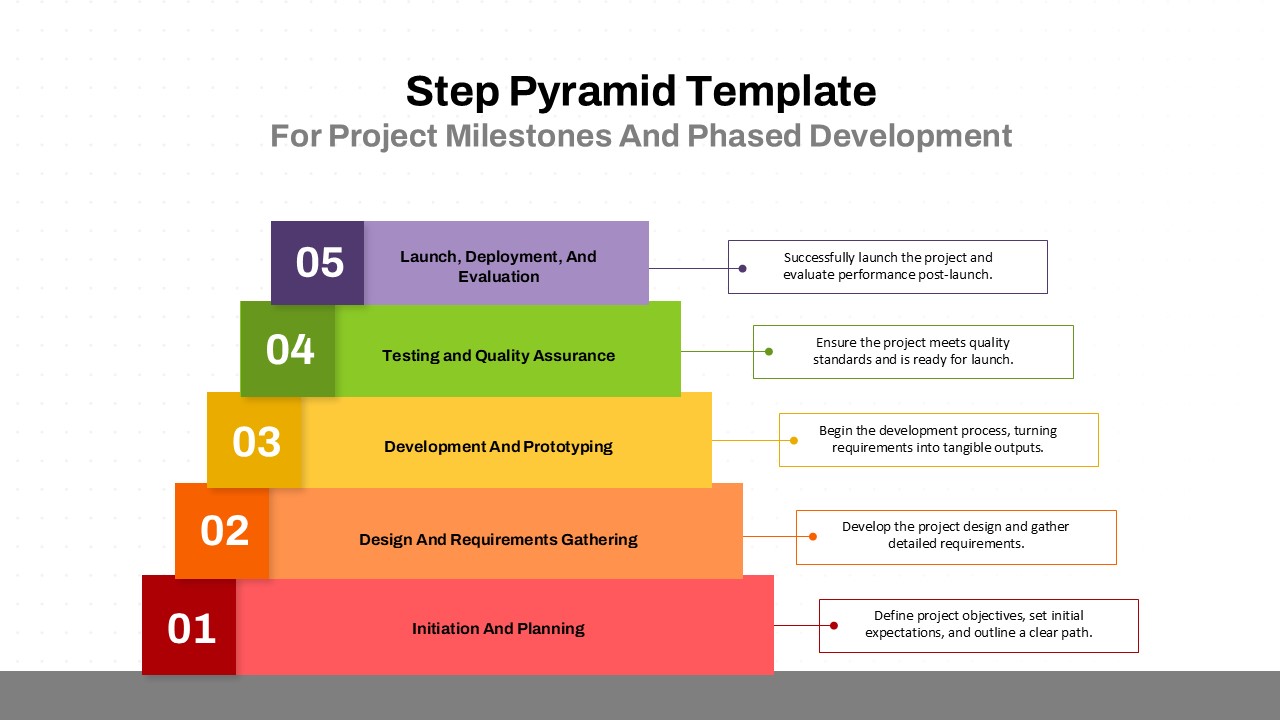
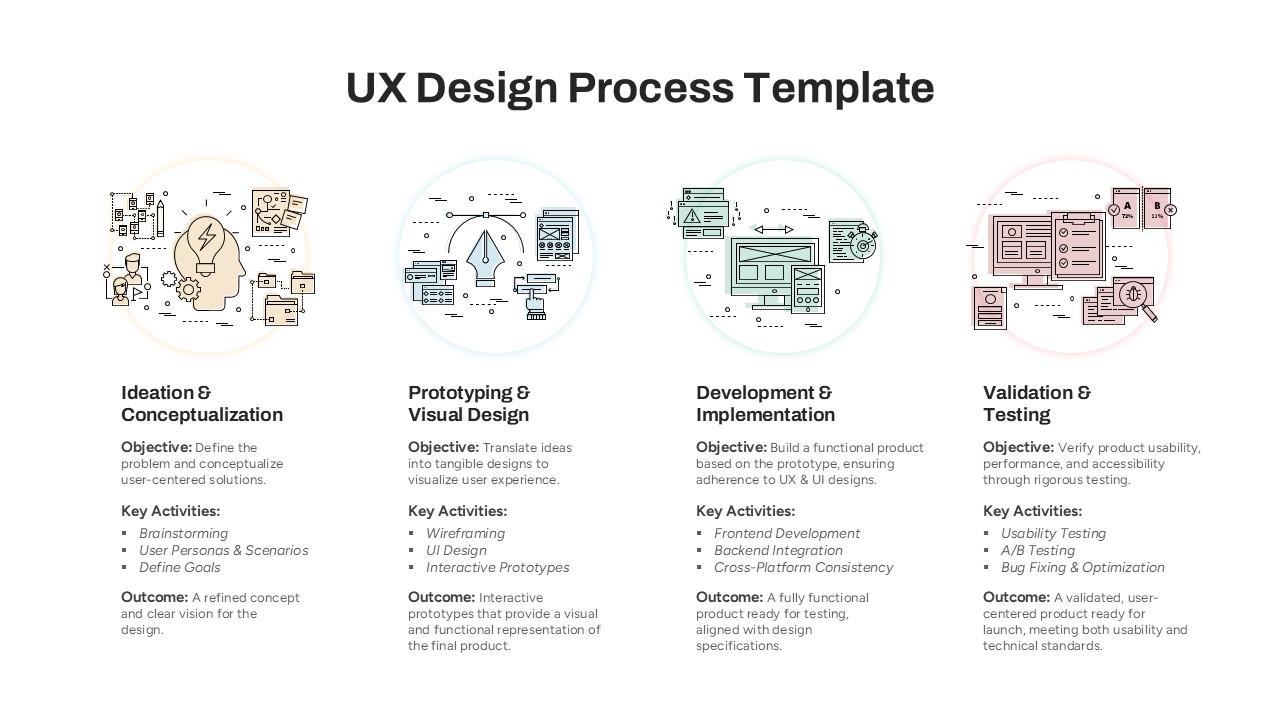
The Clinical Trial Roadmap Template is a professionally crafted slide designed to present the phases of a clinical study in a structured, easy-to-understand format. Ideal for pharmaceutical companies, CROs, and research organizations, this roadmap slide design captures critical milestones from patient recruitment and enrollment to study kick-off, data reporting, and final drug approval.
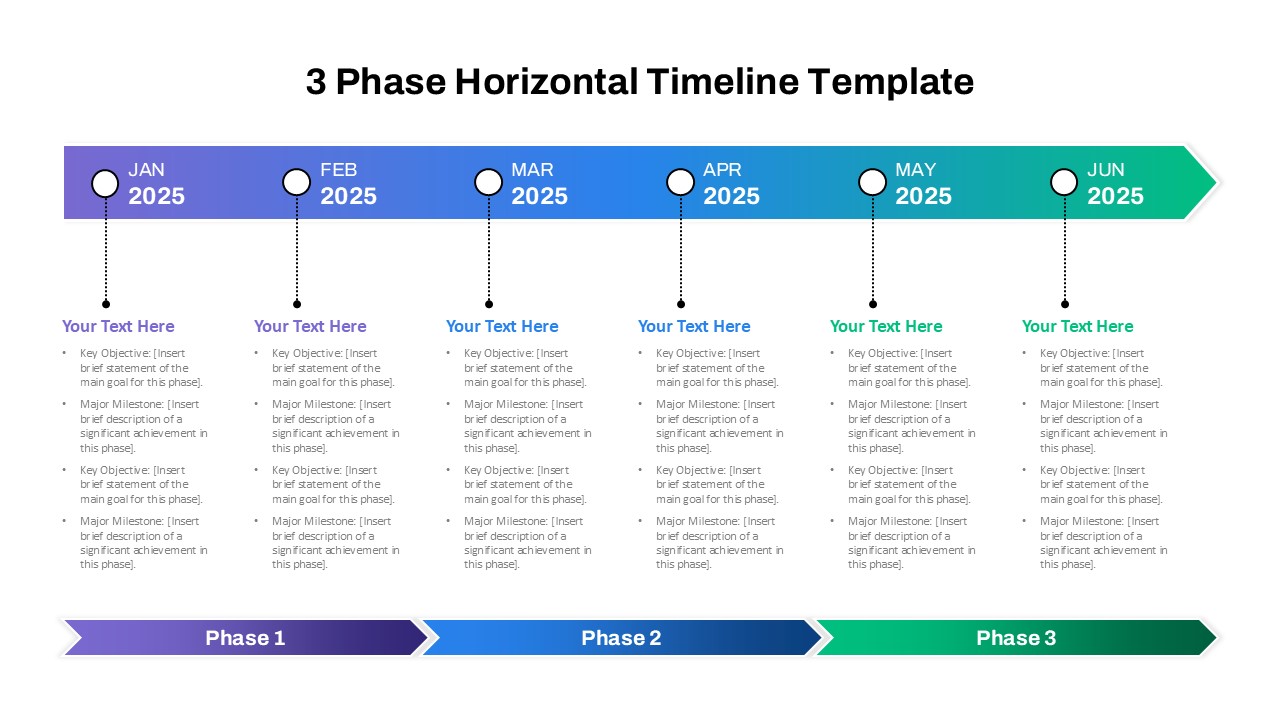
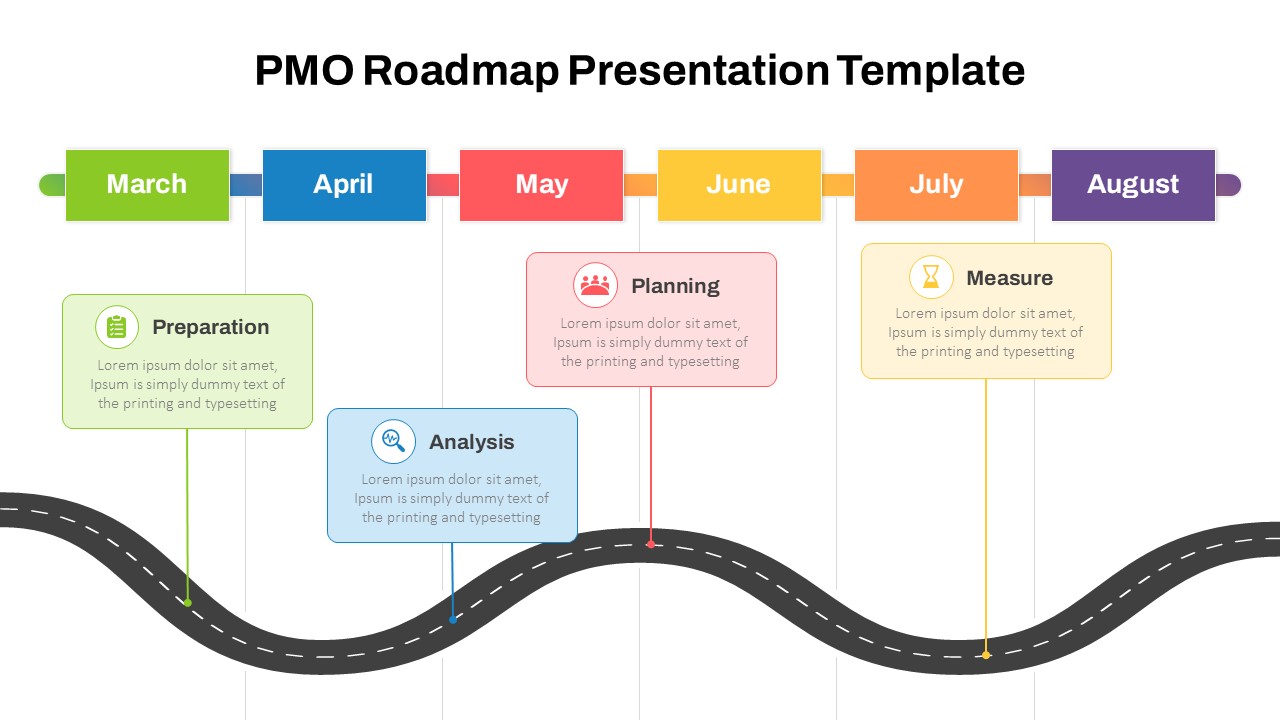
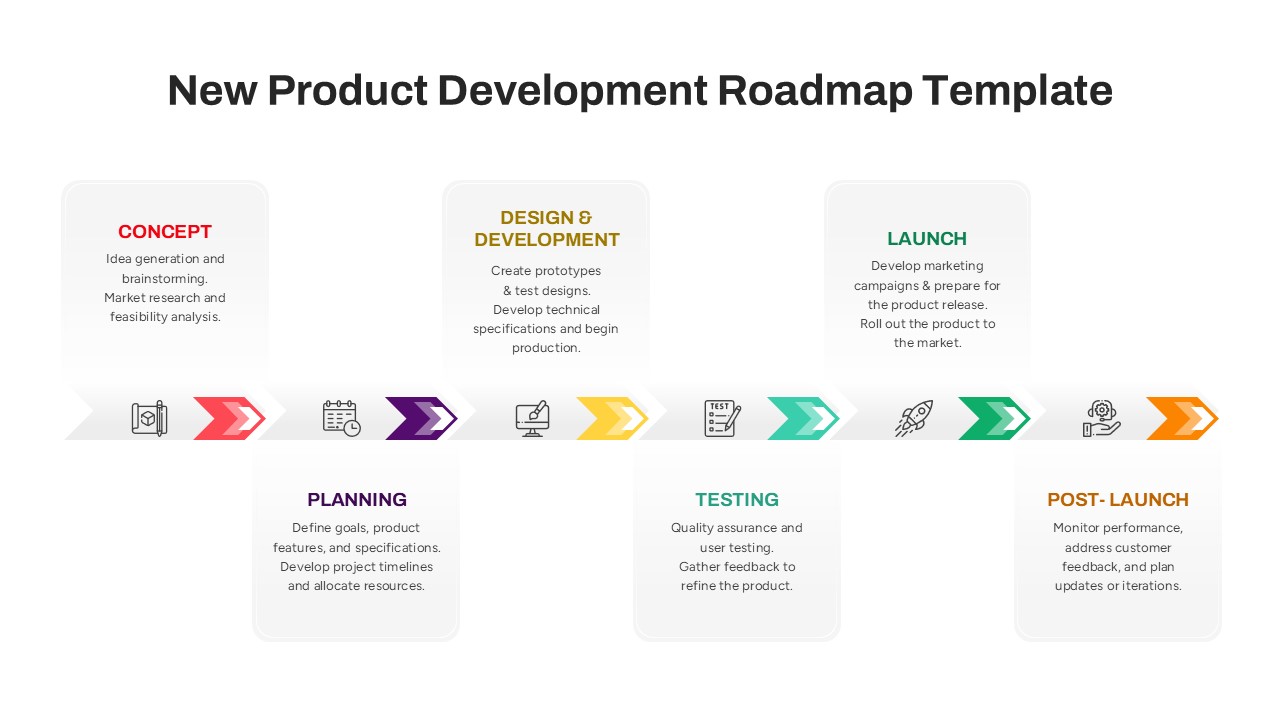
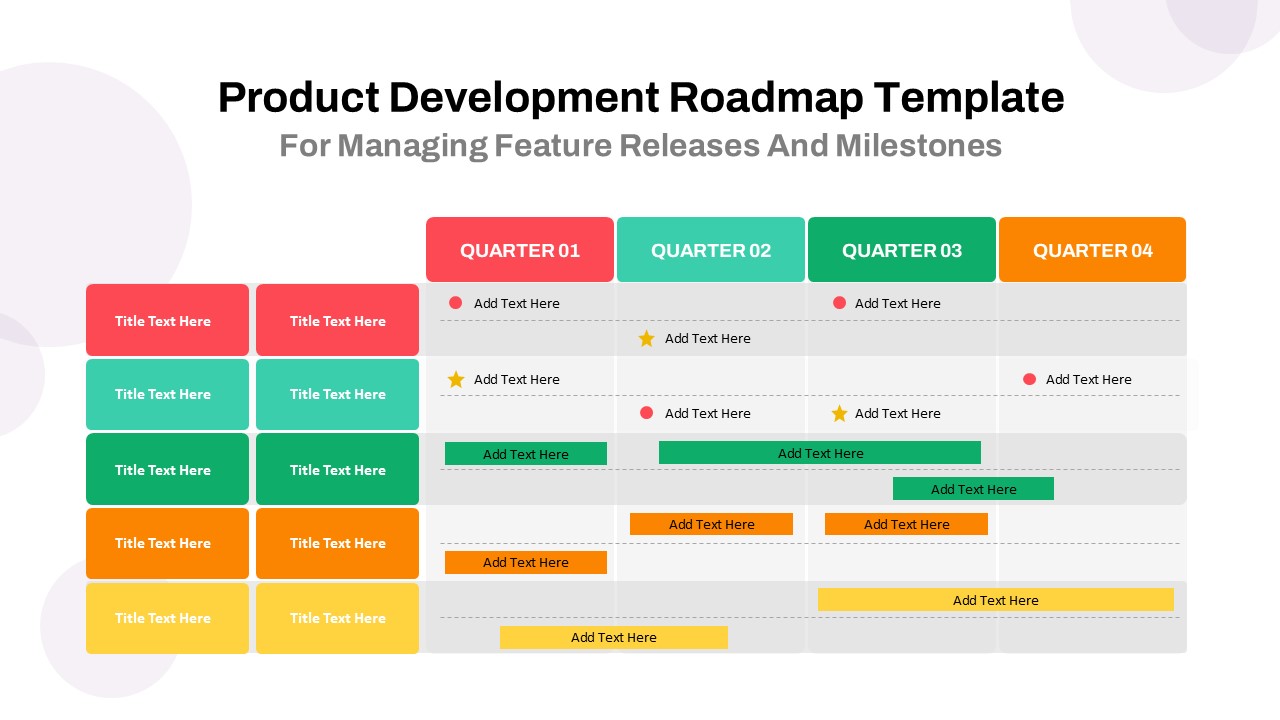
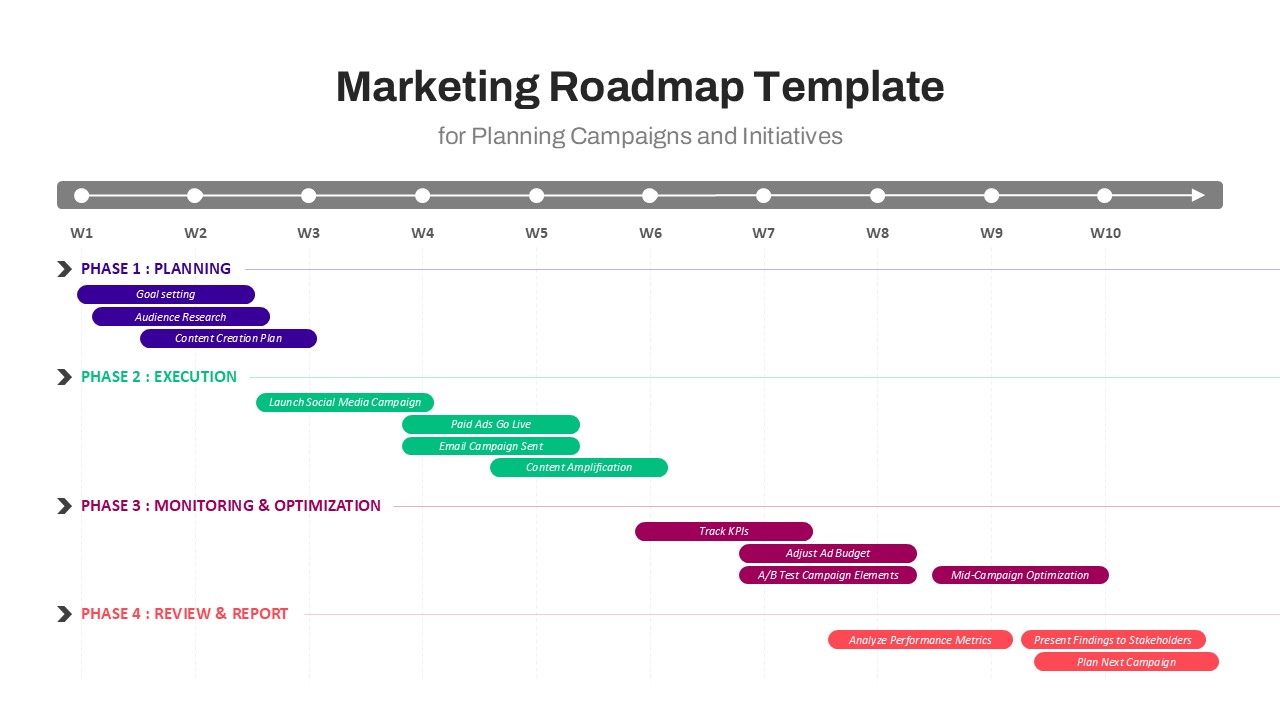
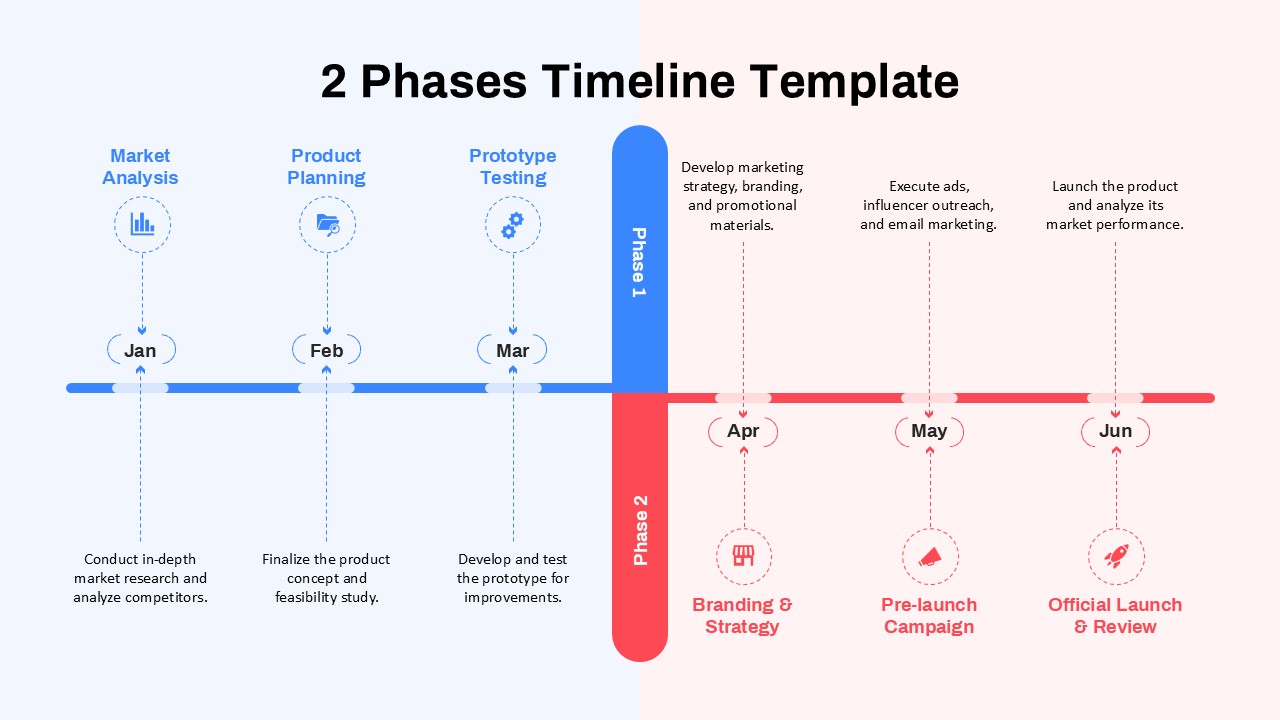
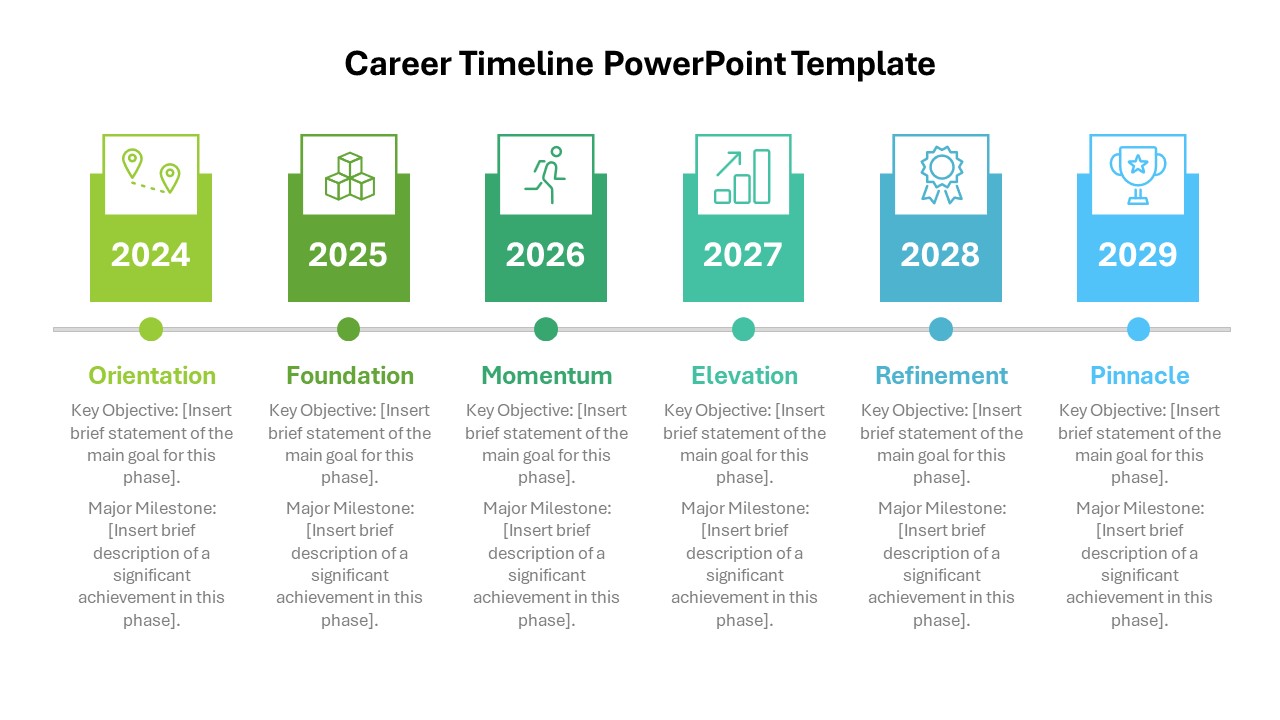
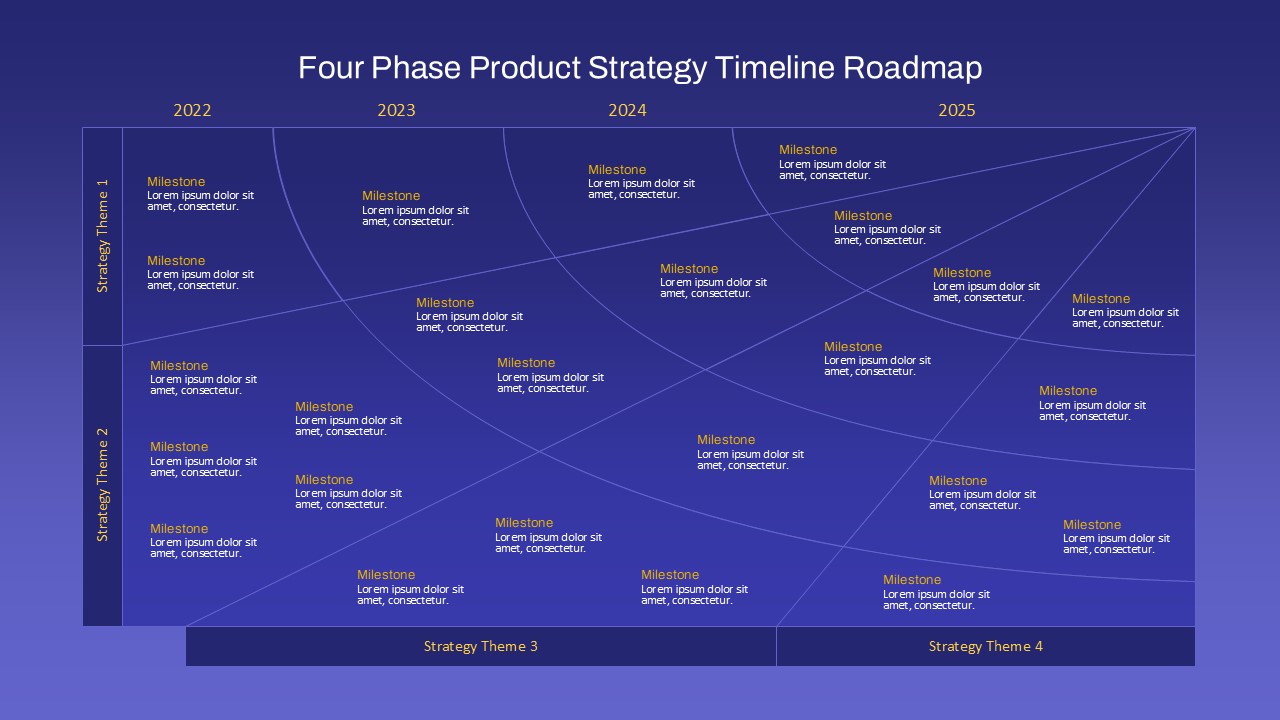
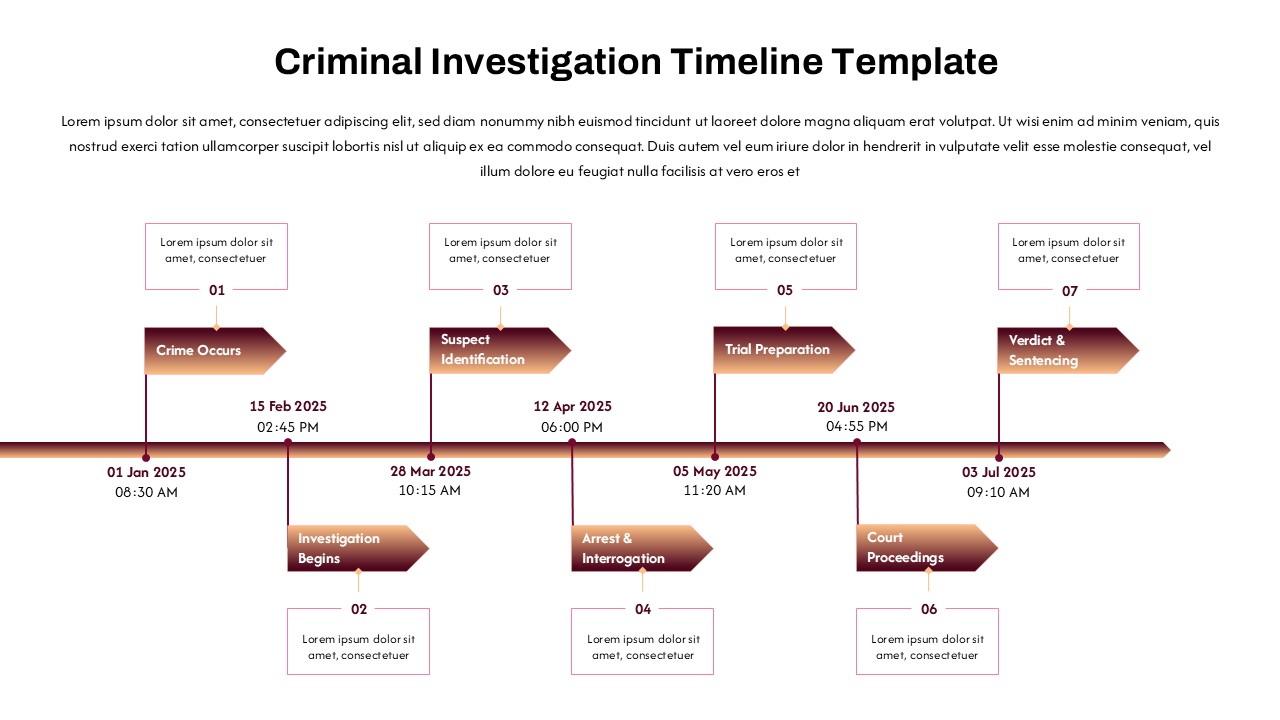
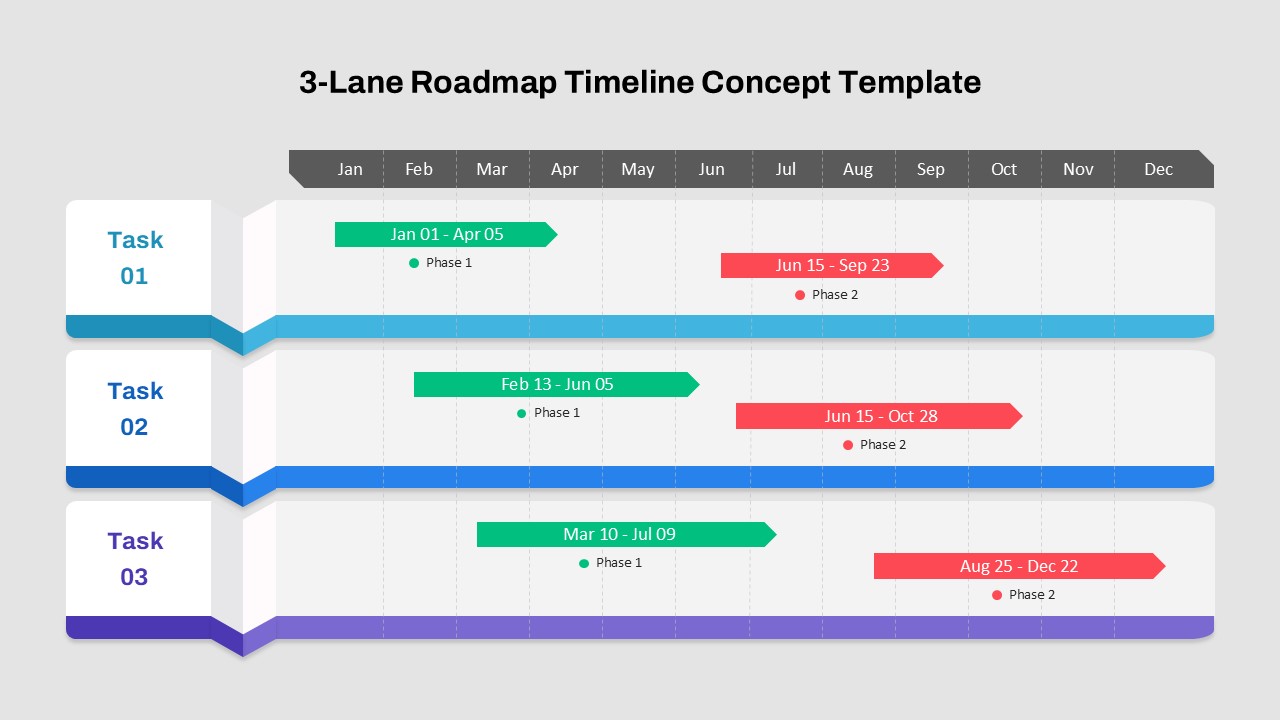
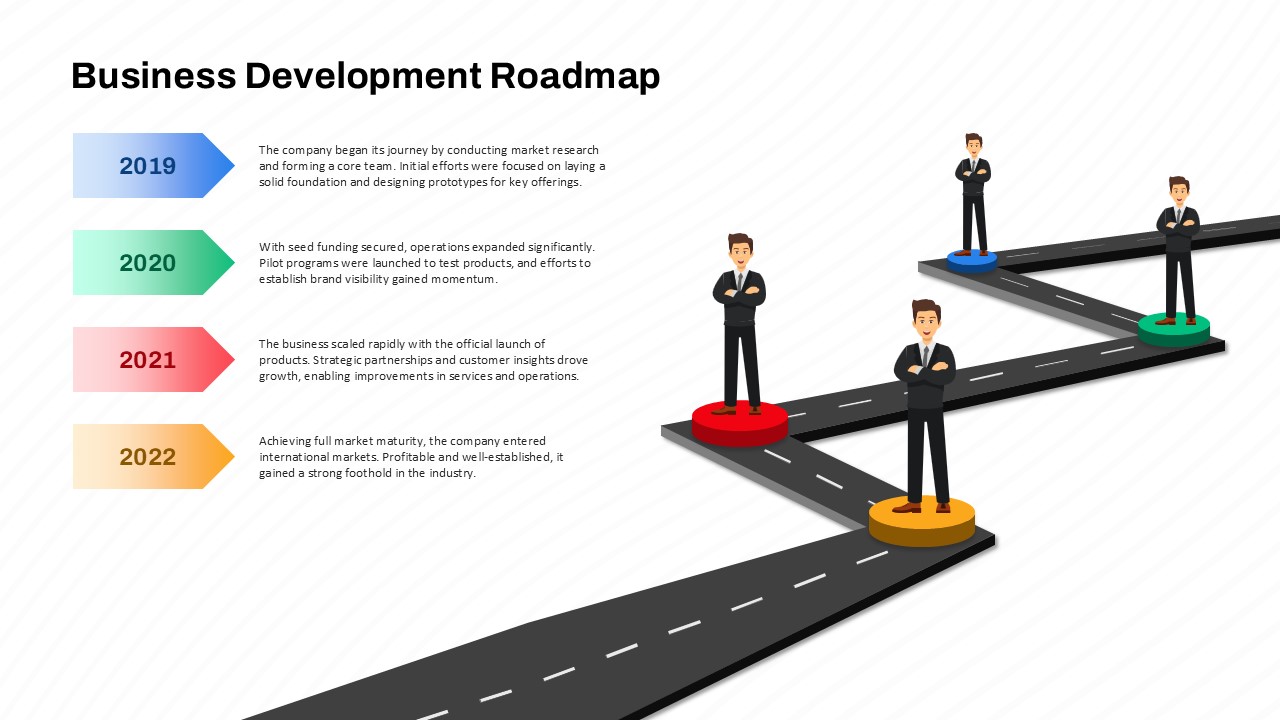
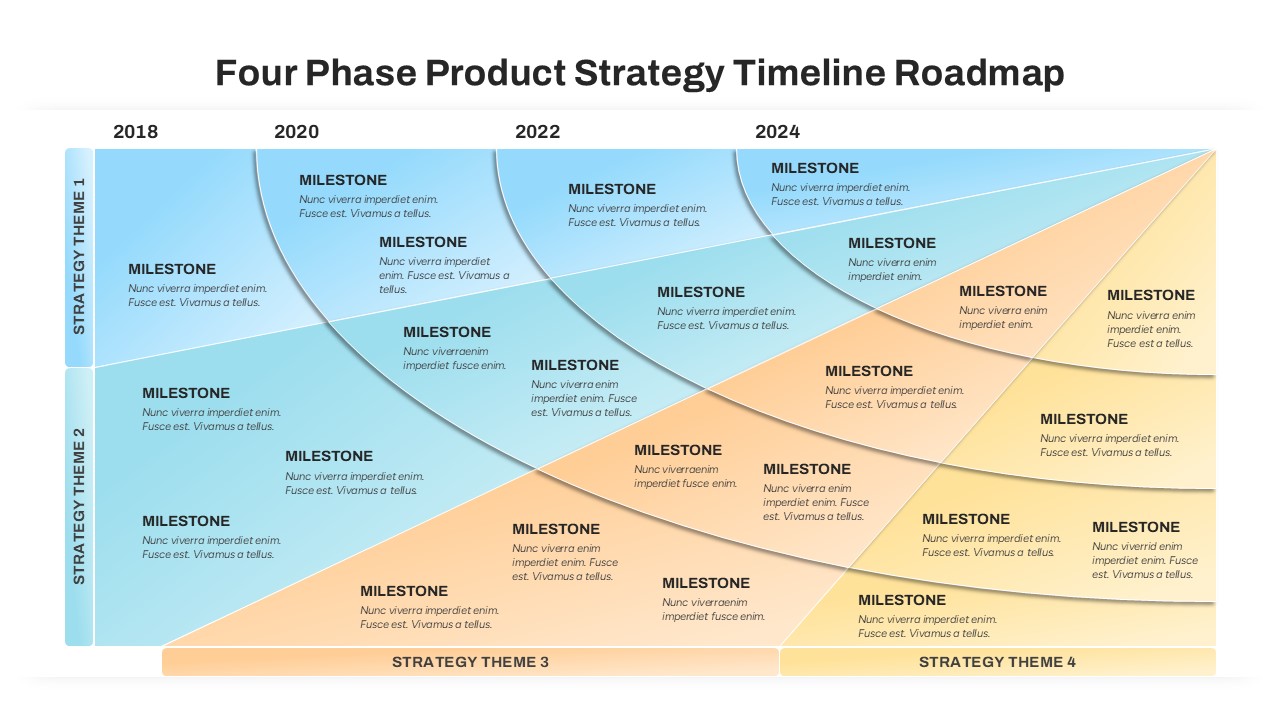
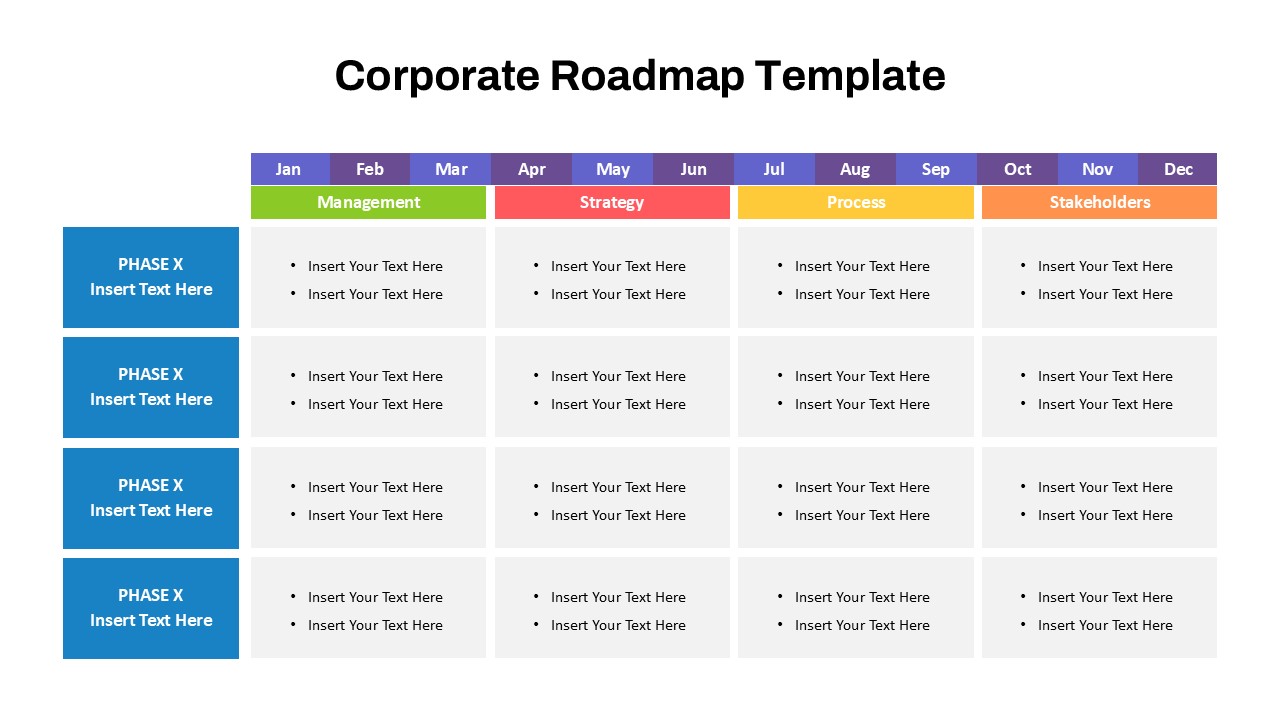
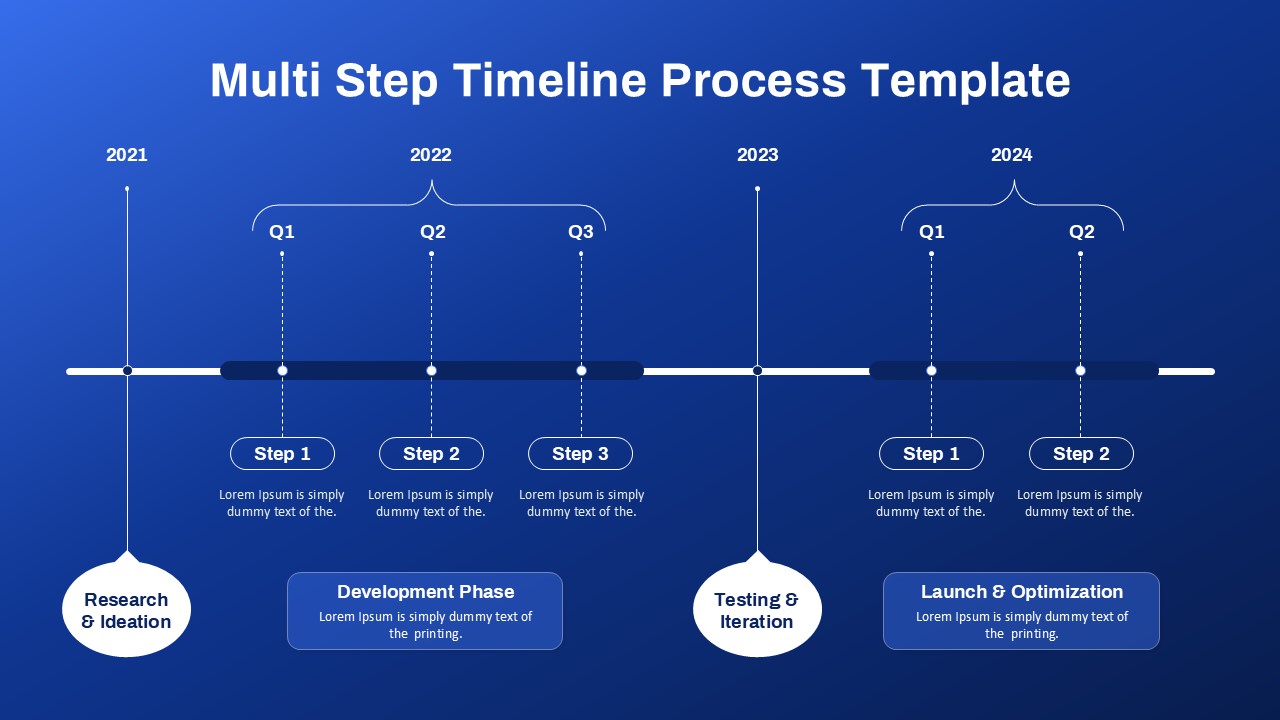
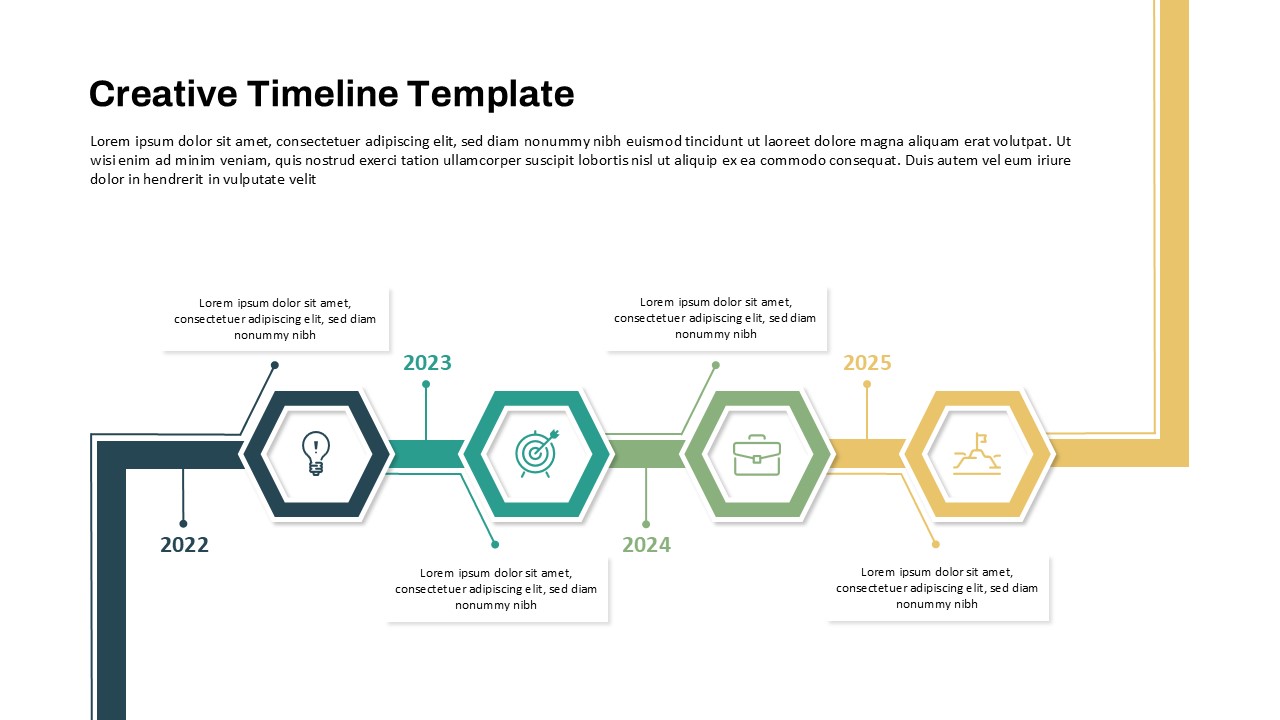
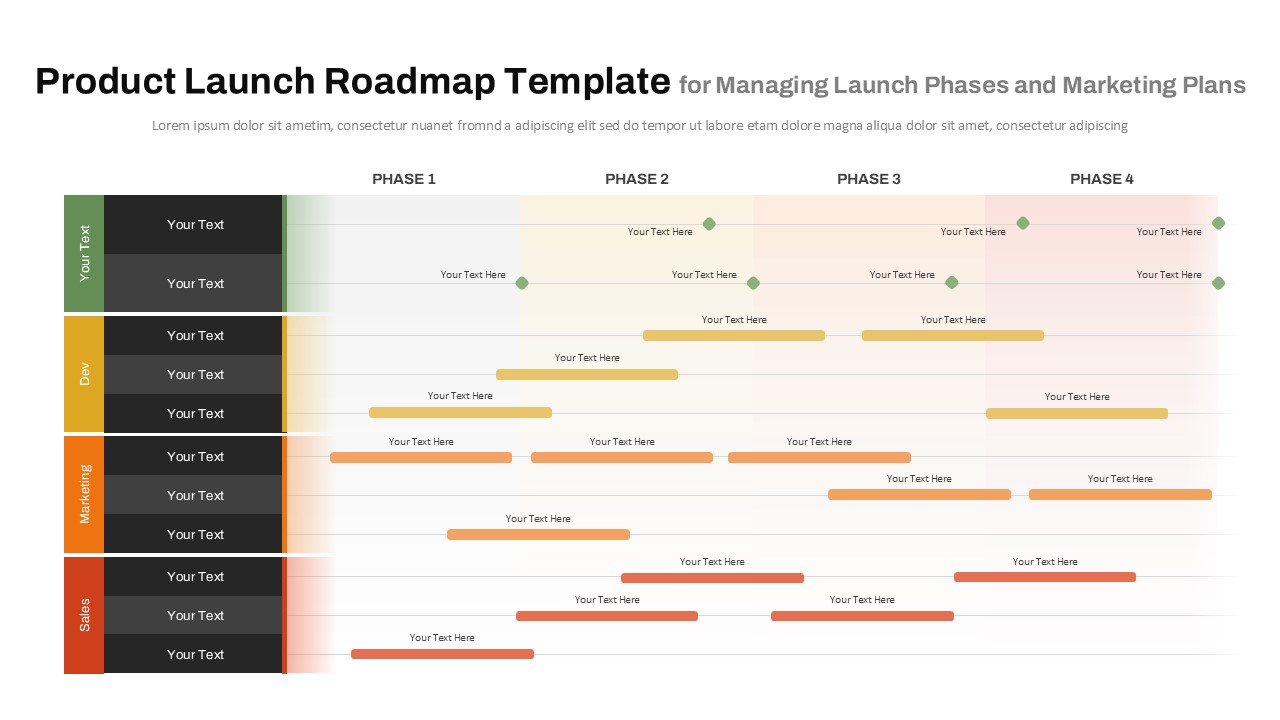
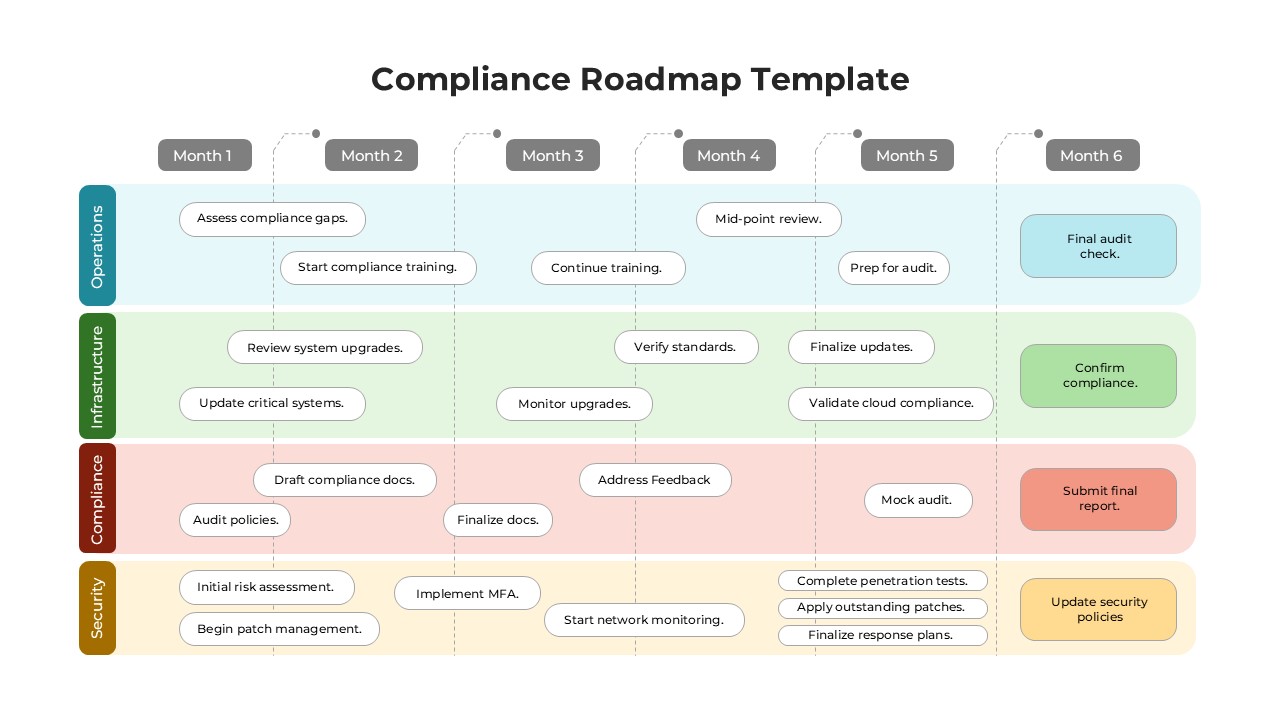
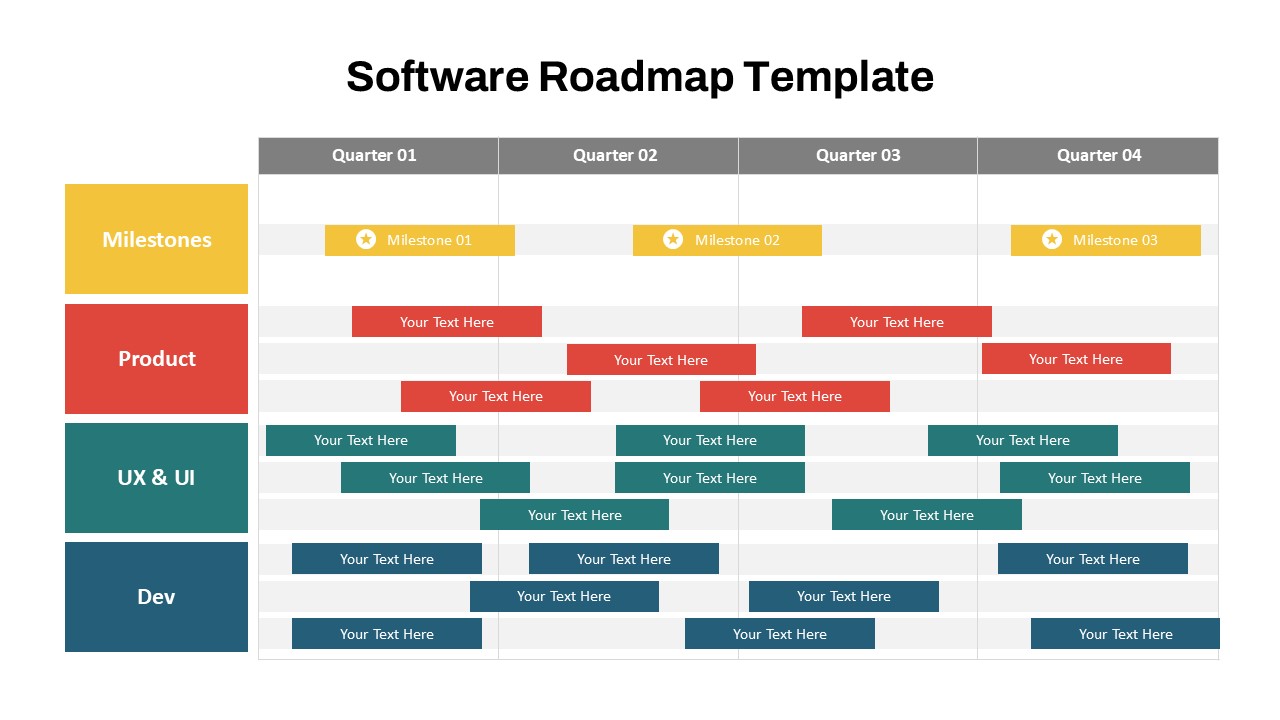
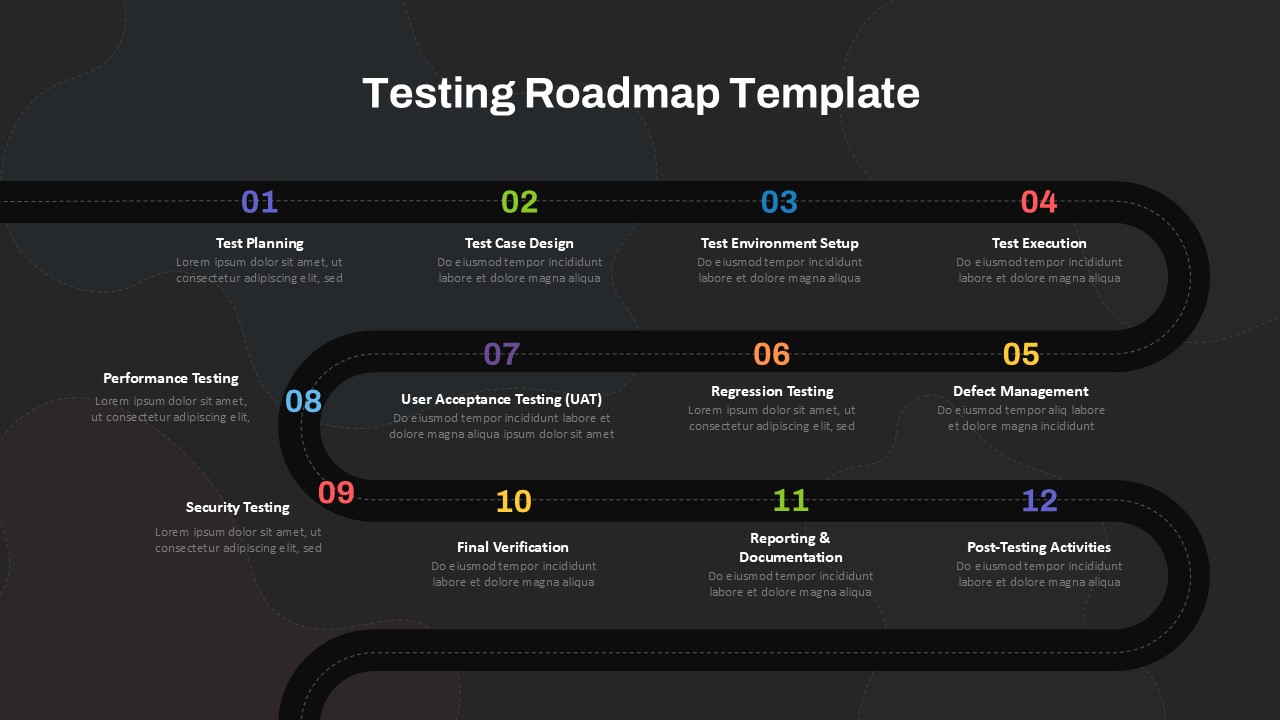
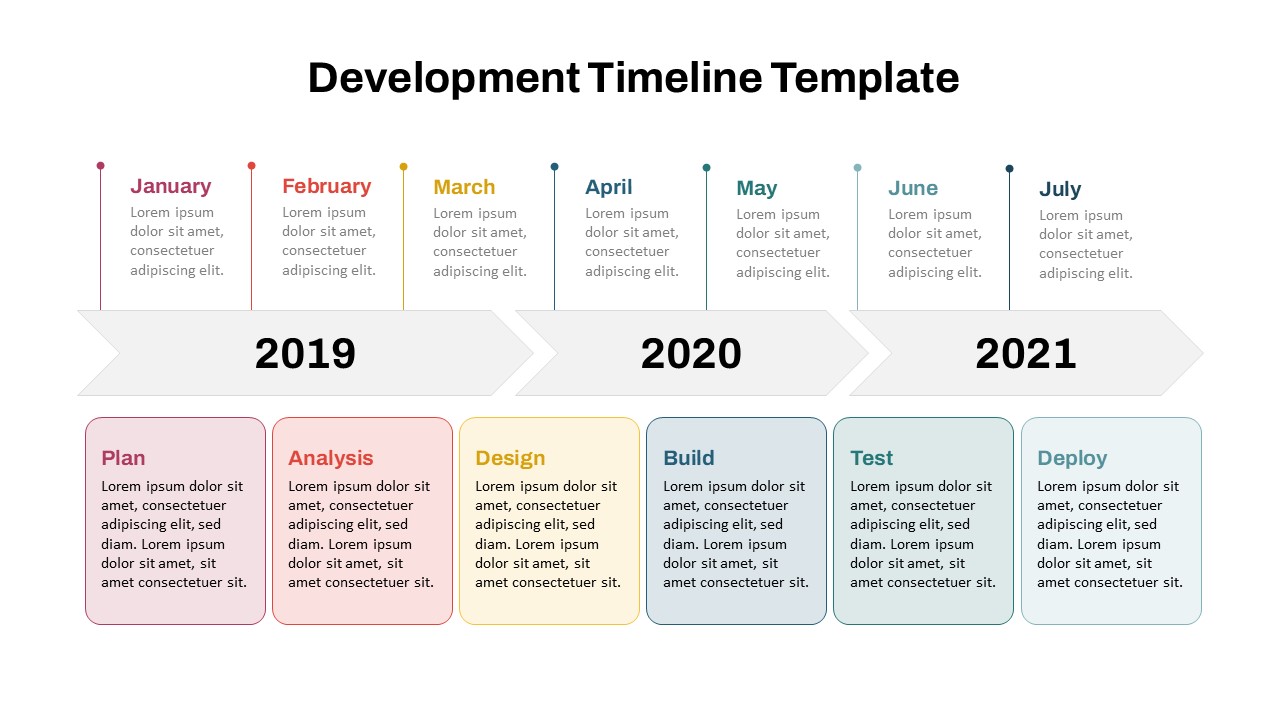
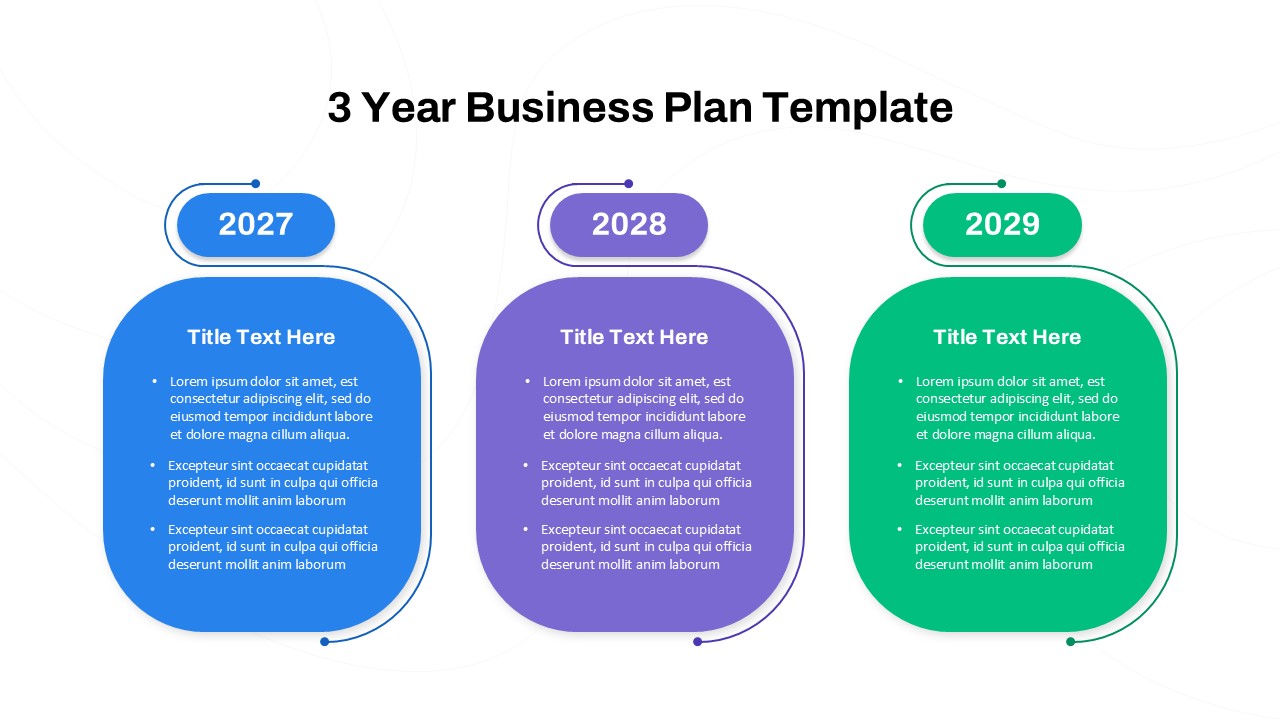
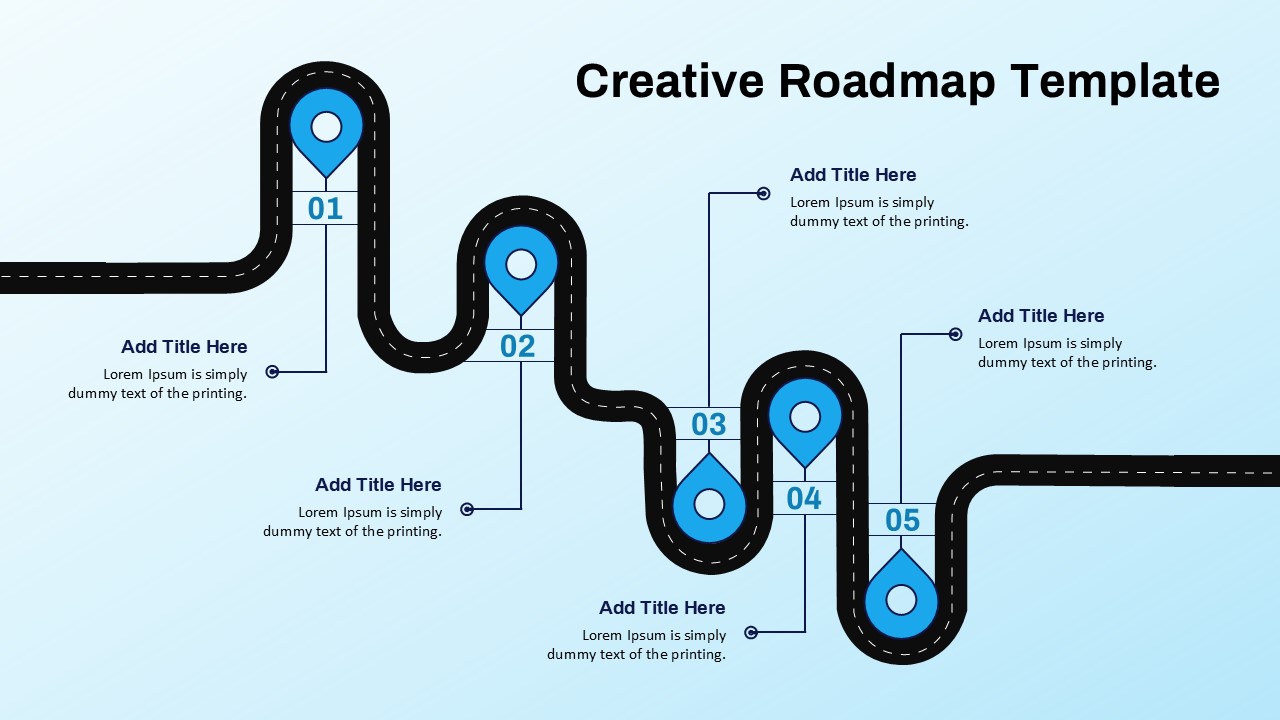
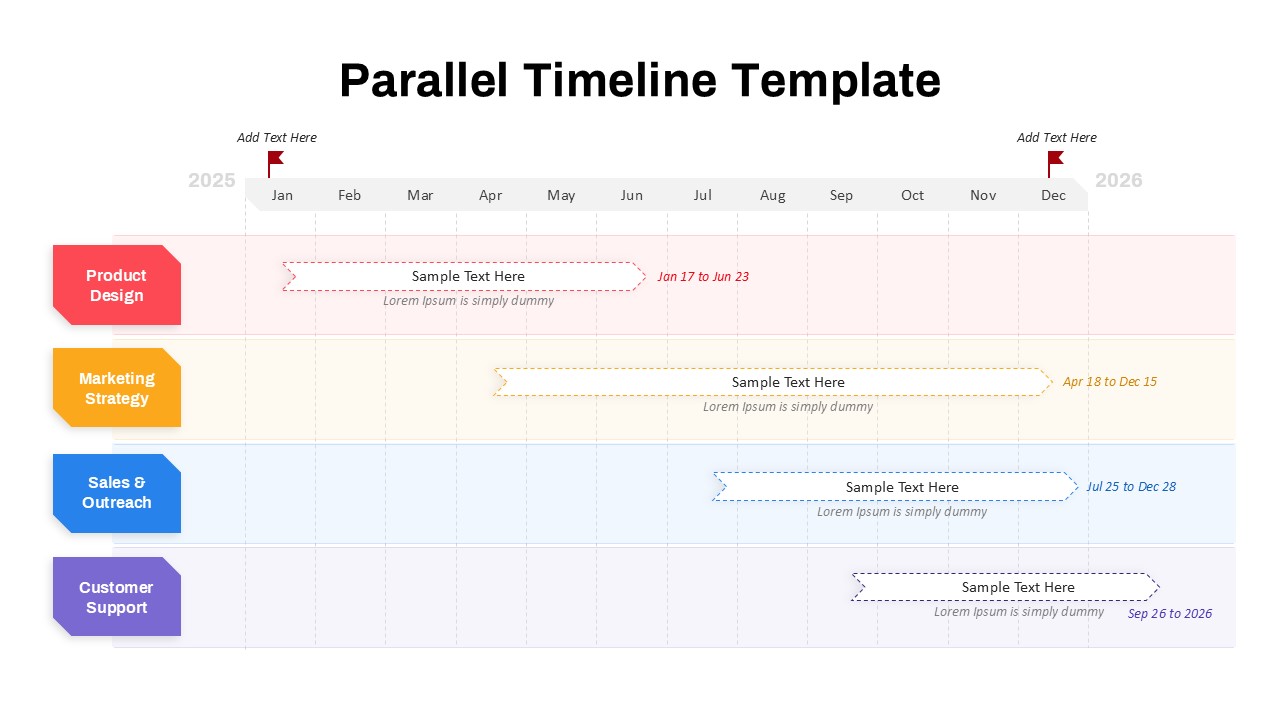
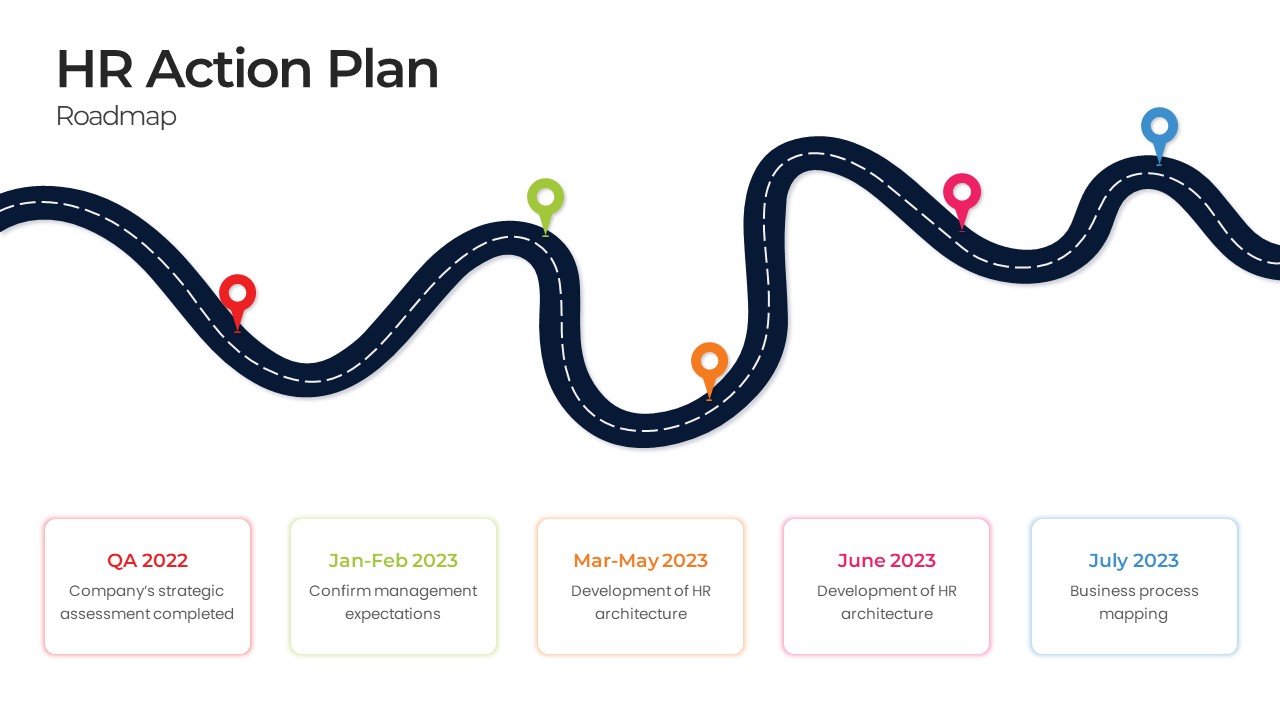
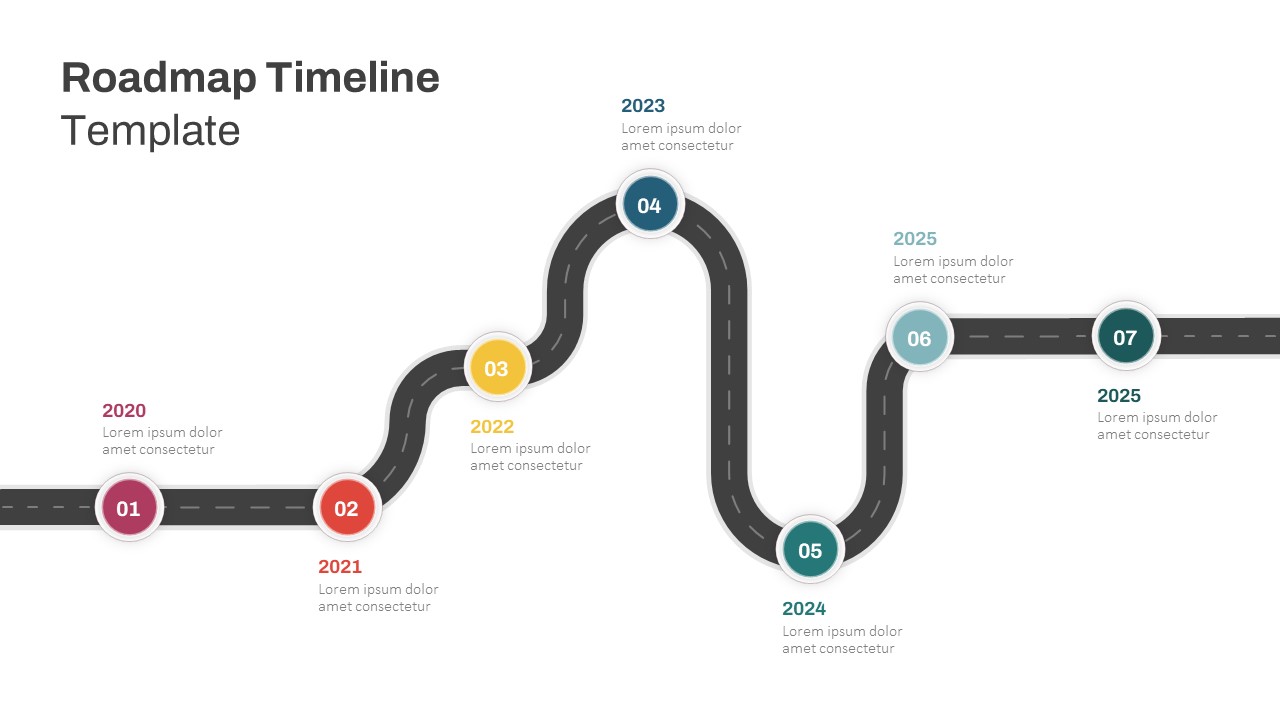
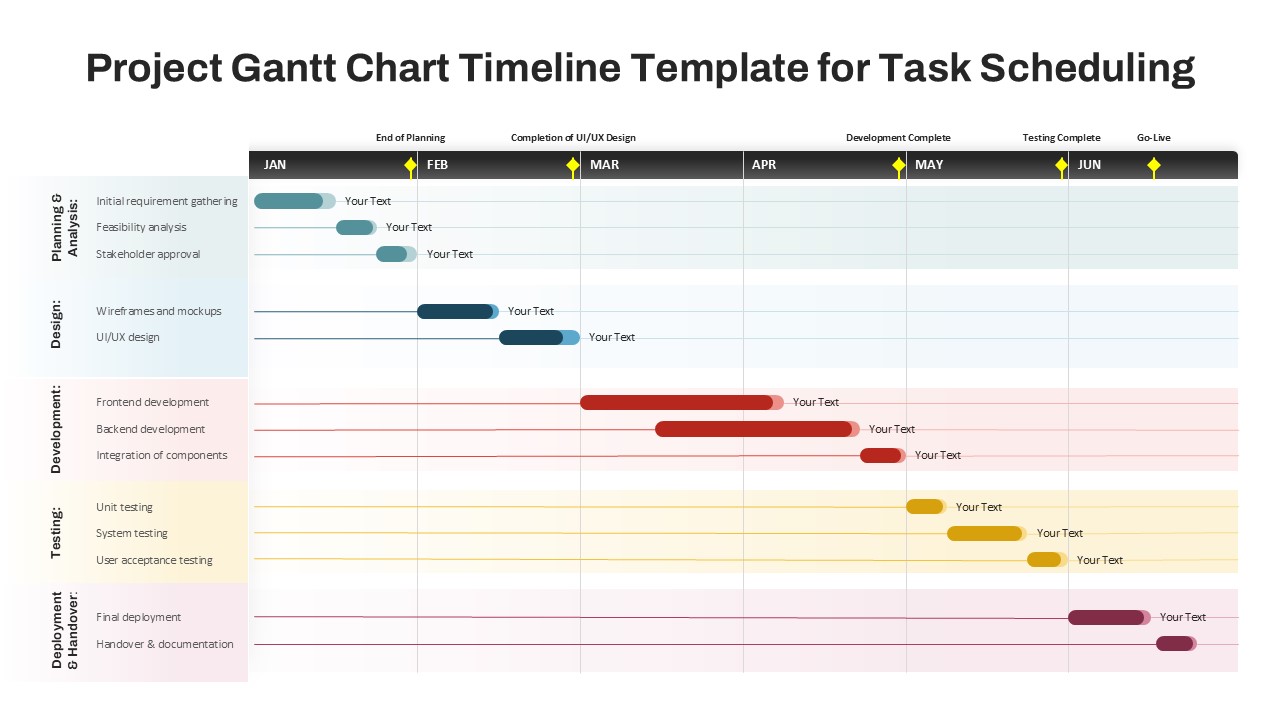
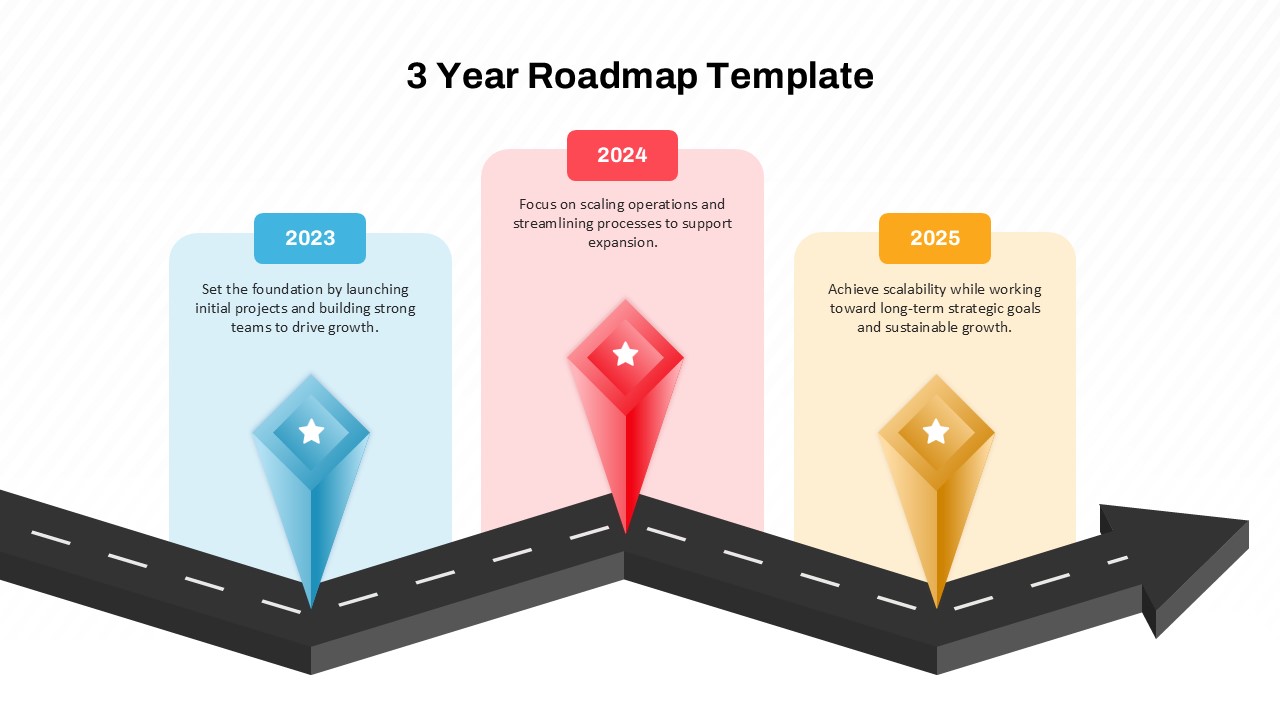
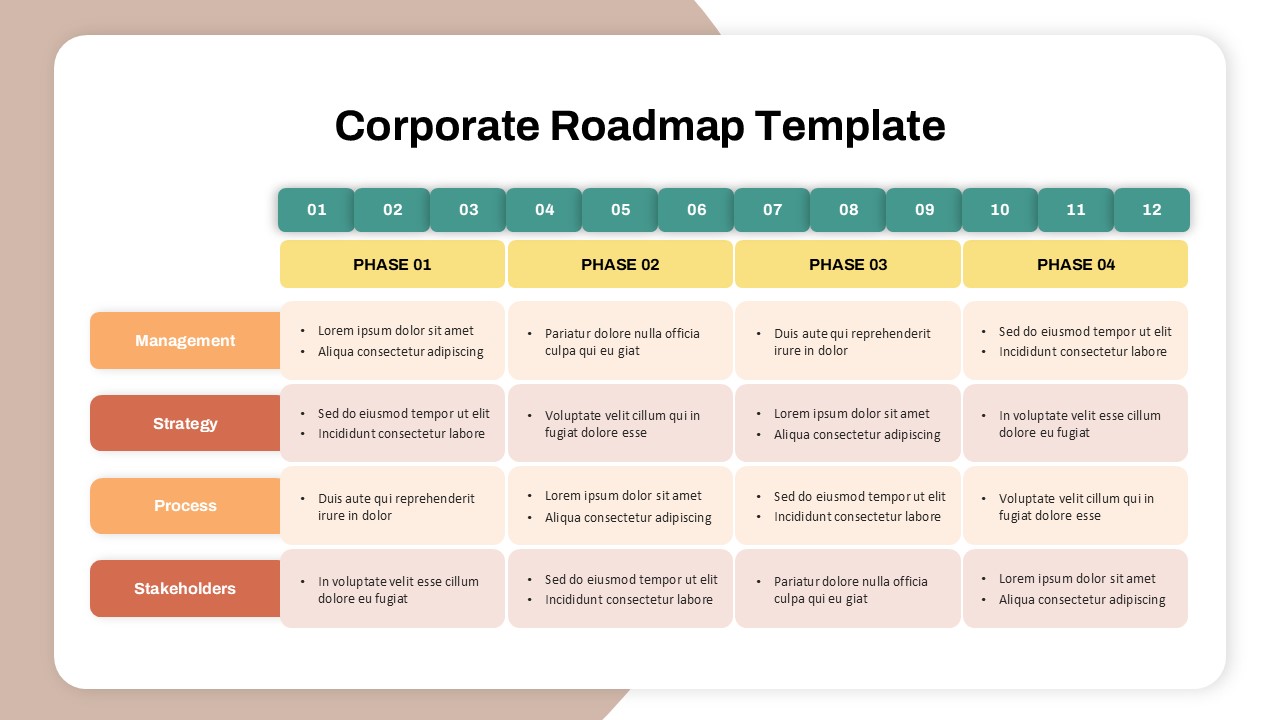
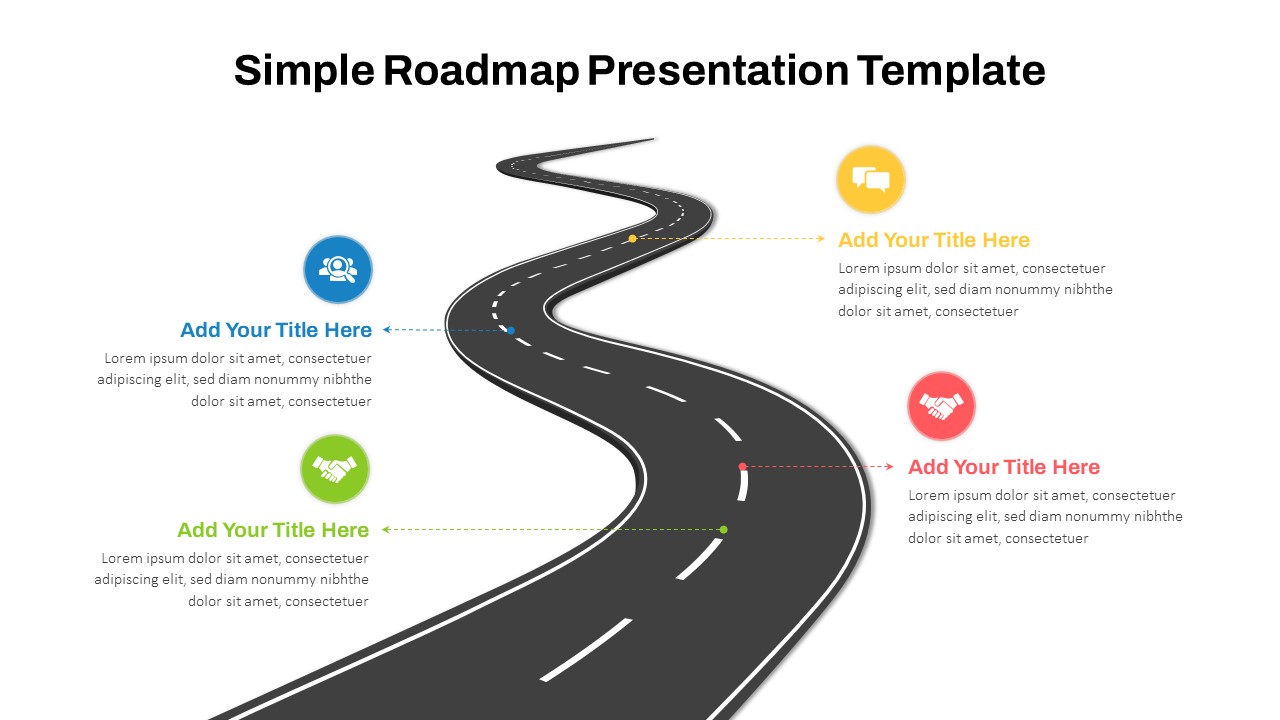
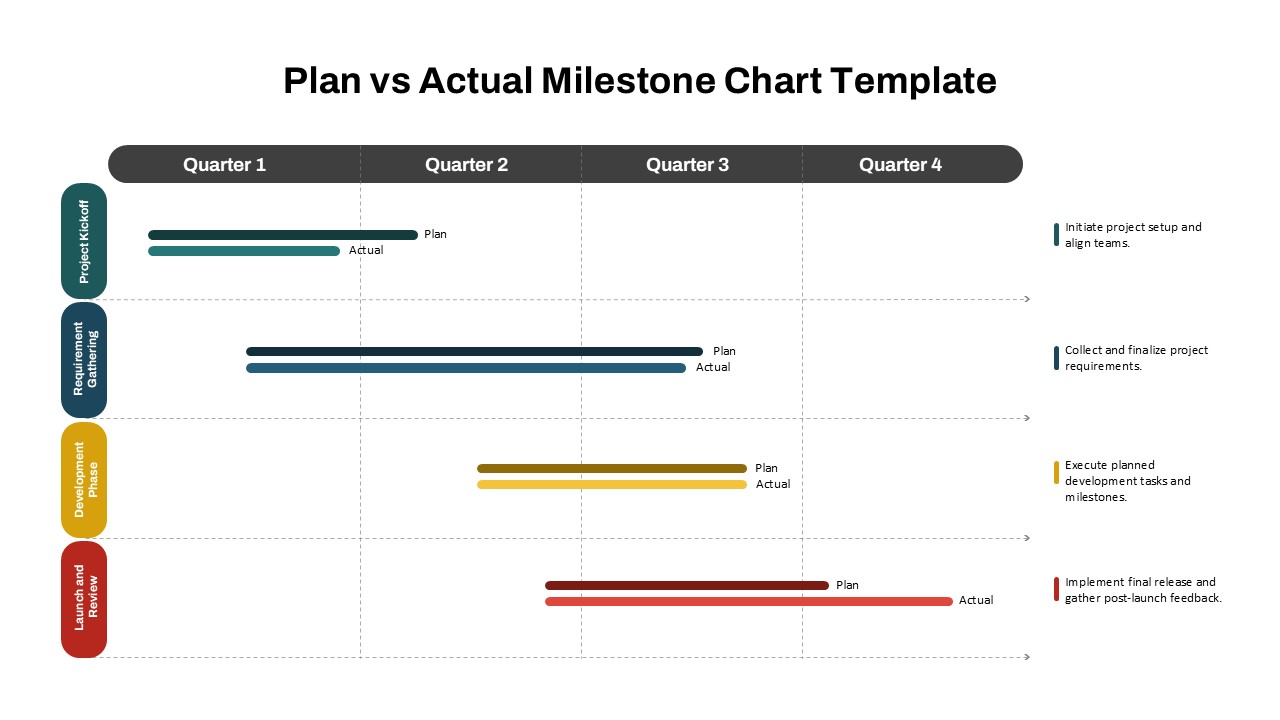
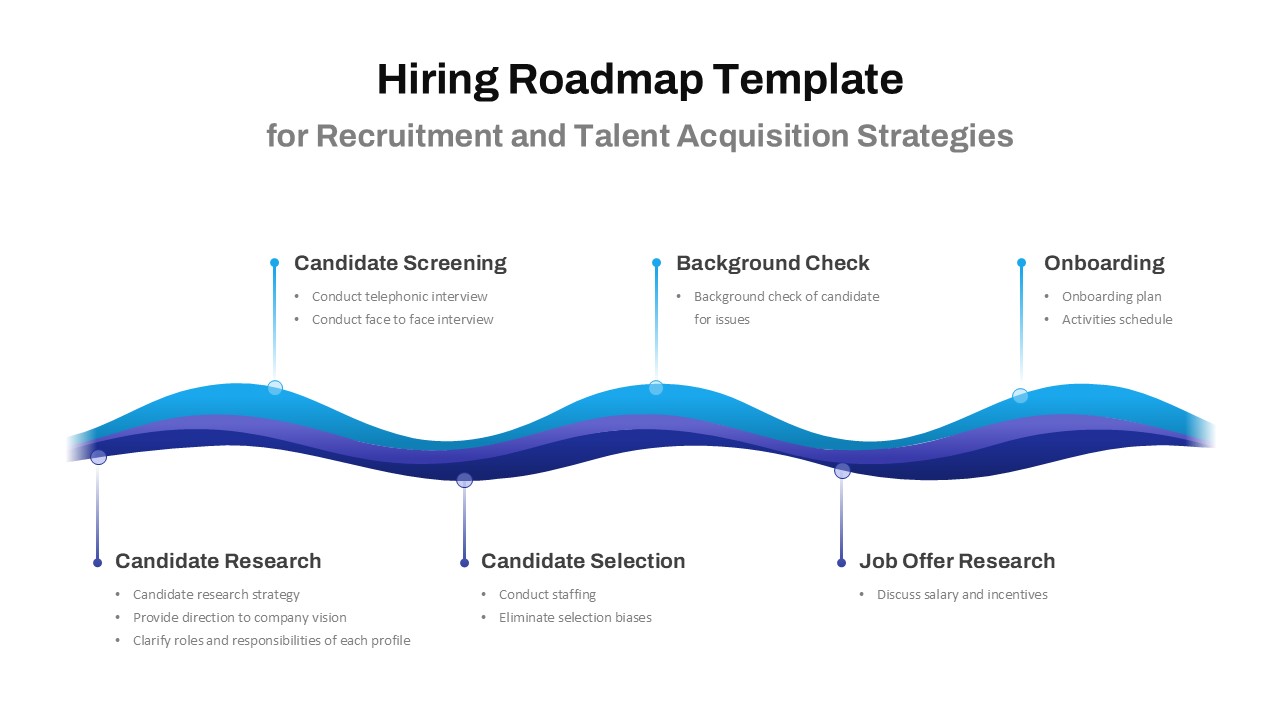
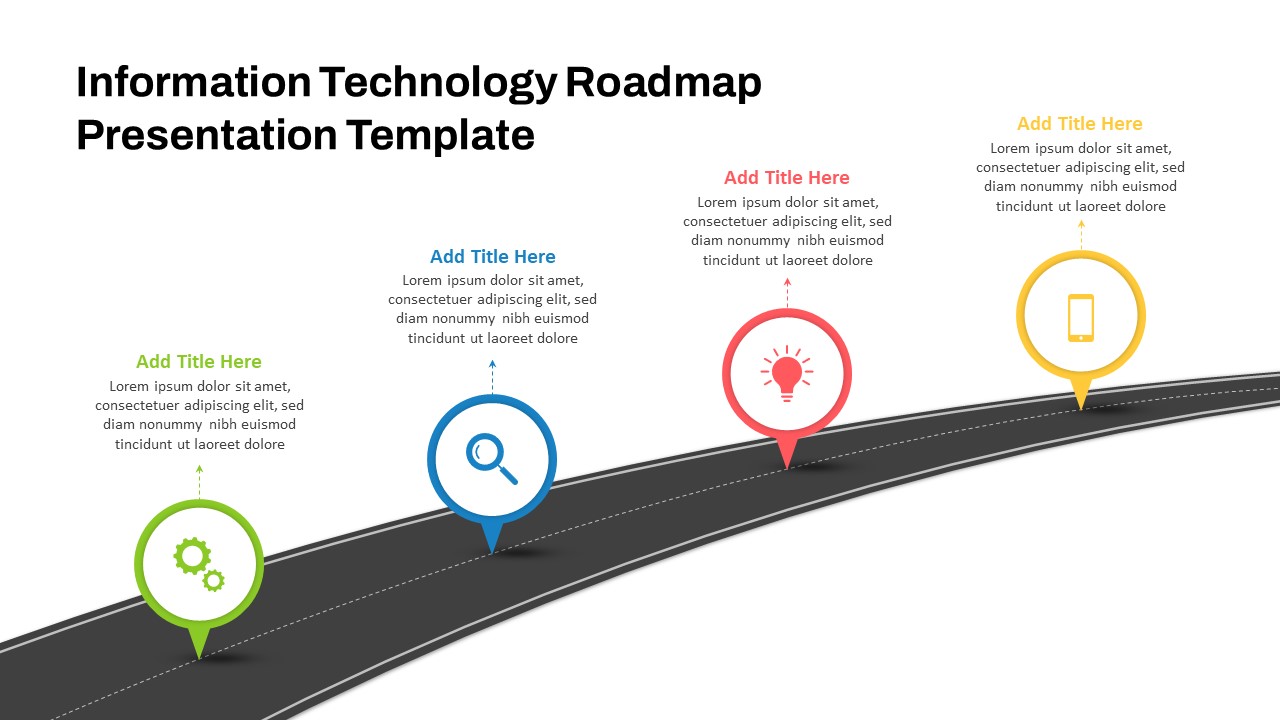
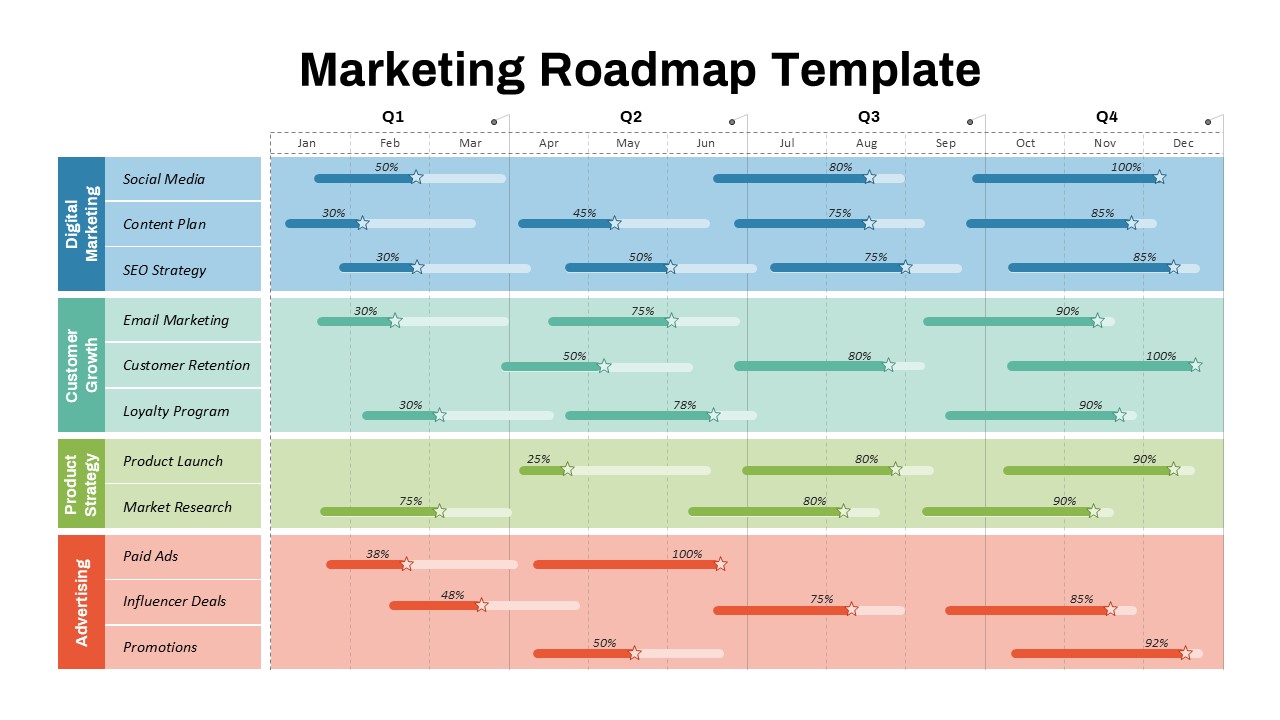
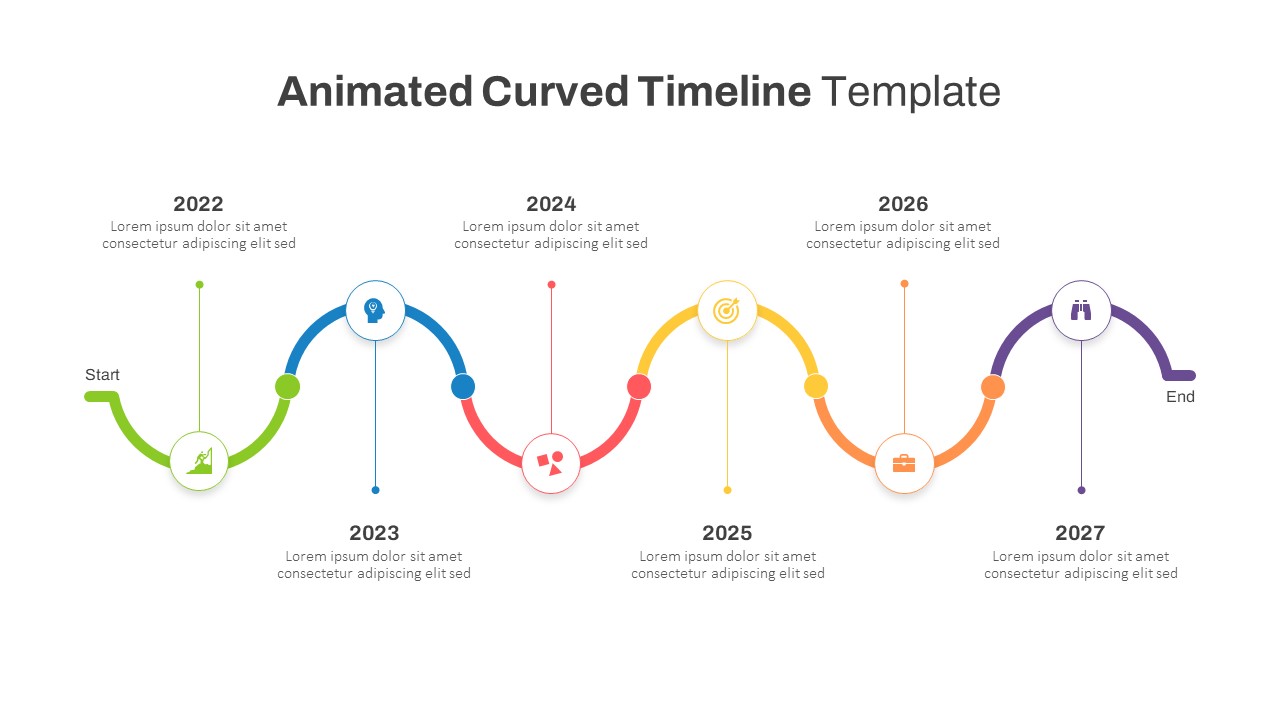
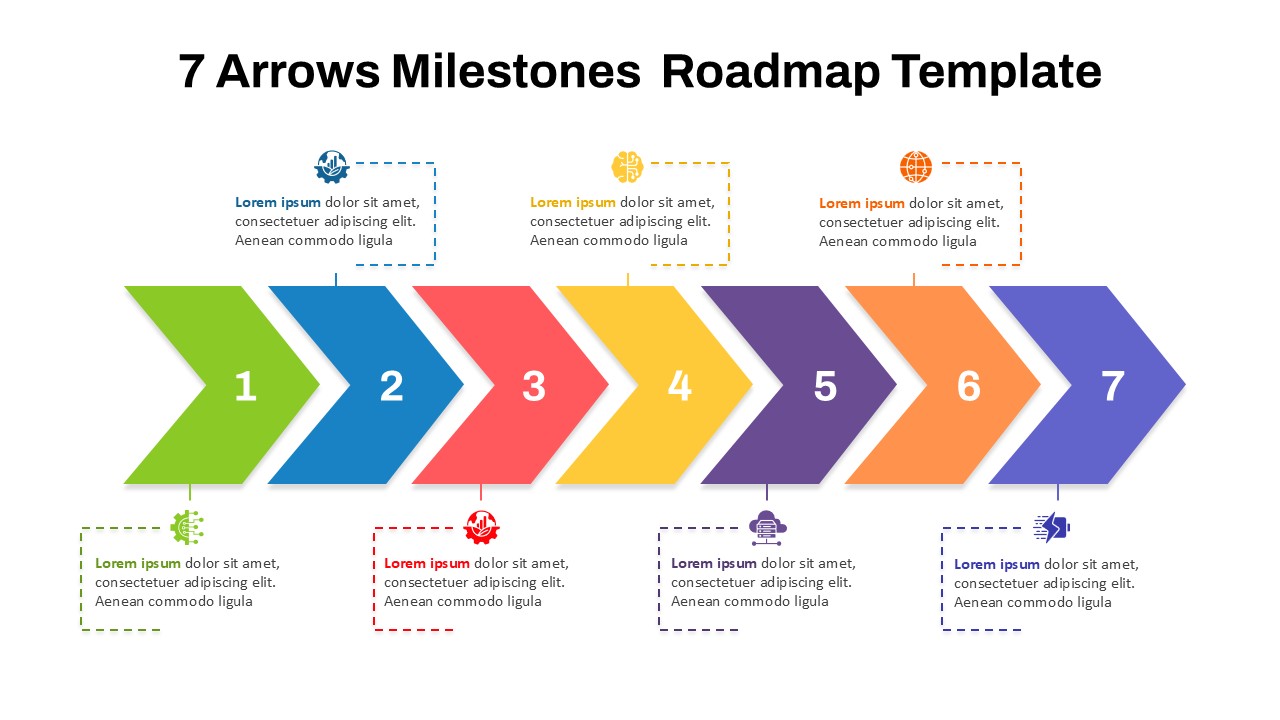
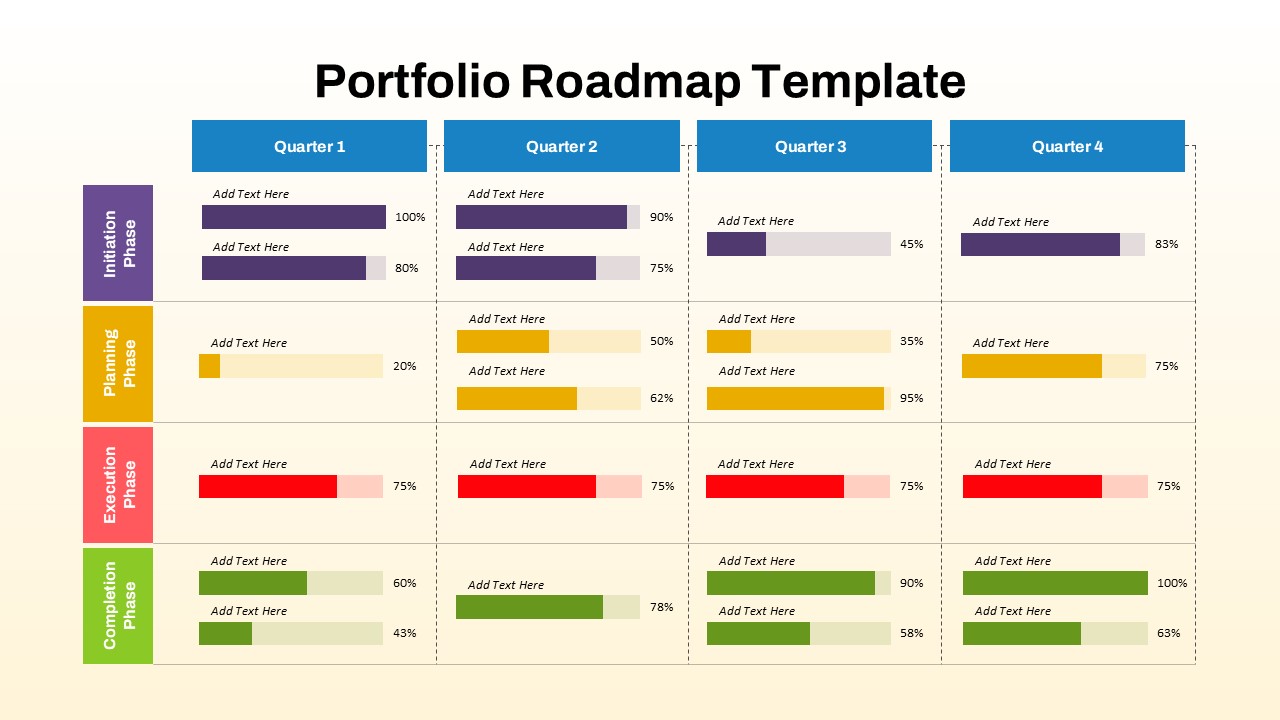
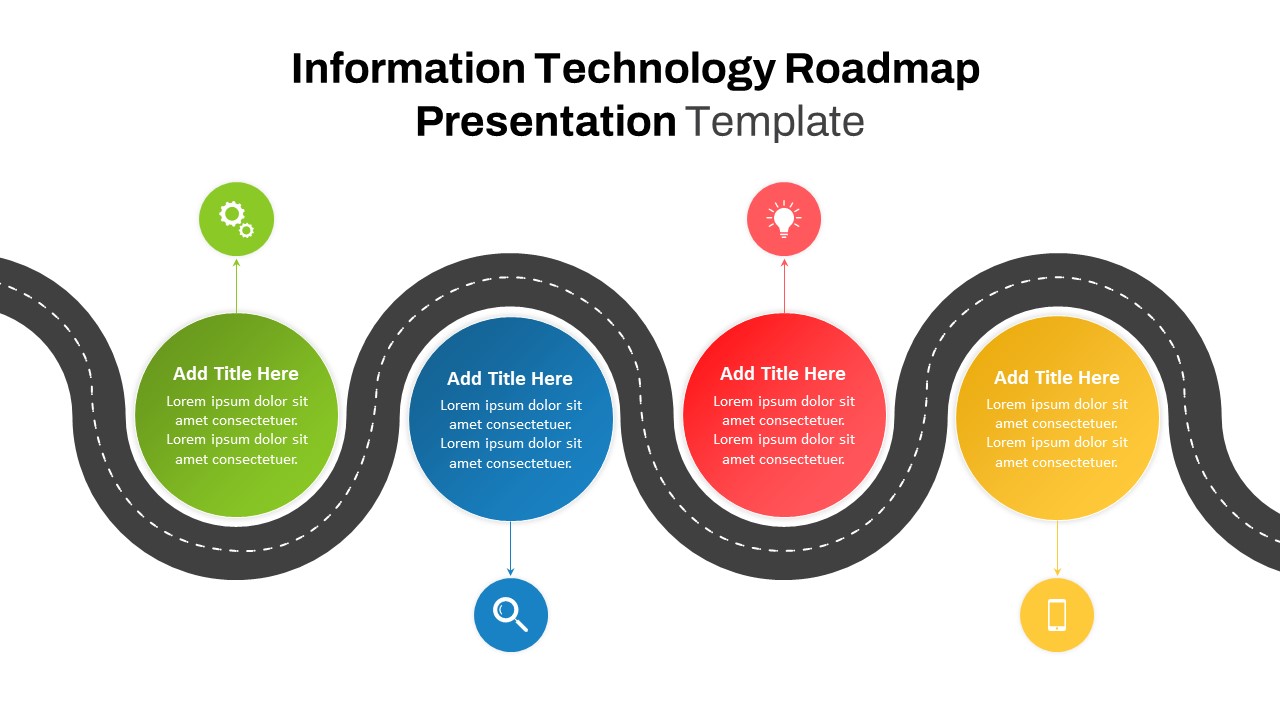
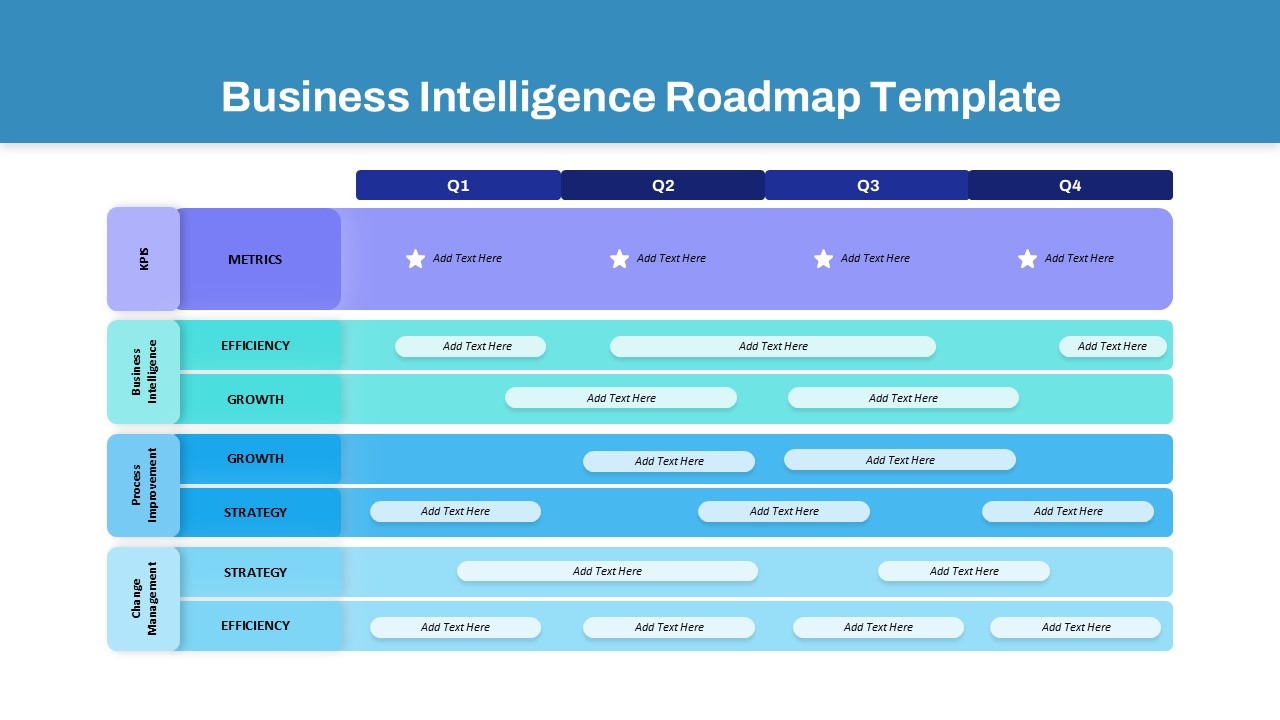
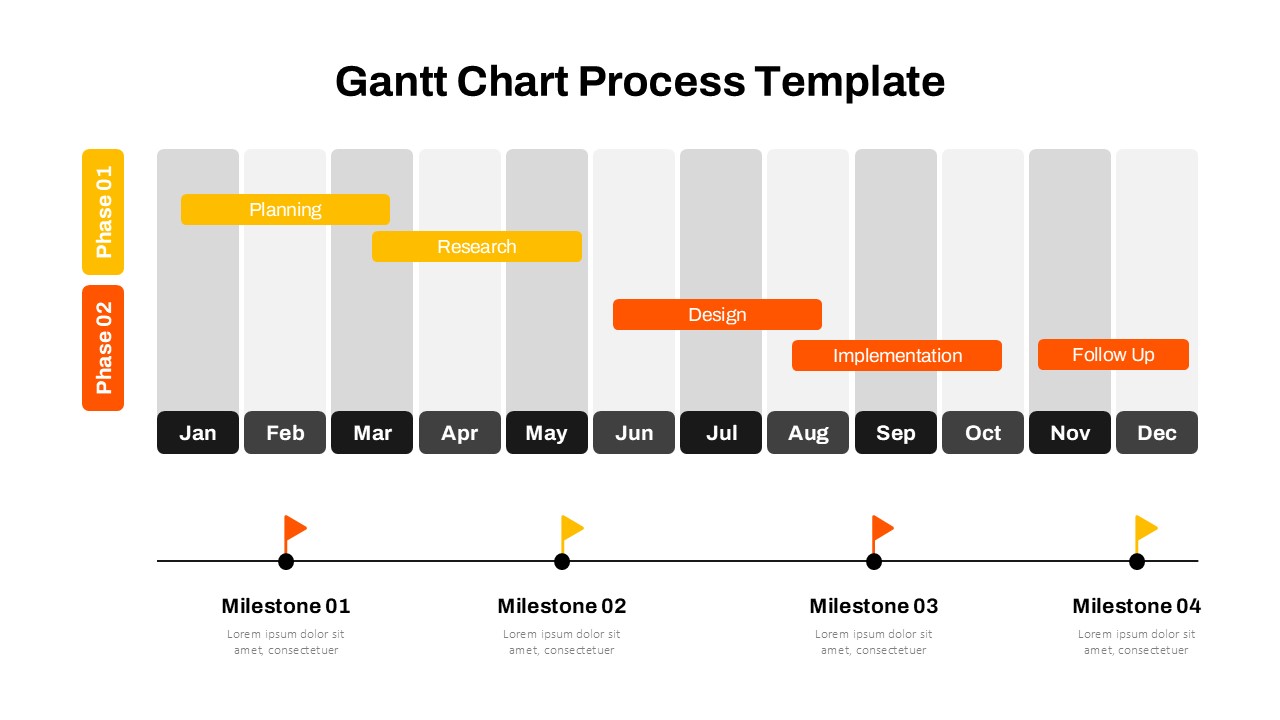
This template showcases three distinct trial phases—Phase 1, Phase 2, and Phase 3—using a horizontal timeline and visually segmented arrows. Each phase includes clearly marked events with dates and color-coded indicators to help communicate the clinical journey effectively. The timeline spans from early 2025 to the end of 2026, making it a comprehensive roadmap format PowerPoint example that aligns well with industry reporting and regulatory expectations.
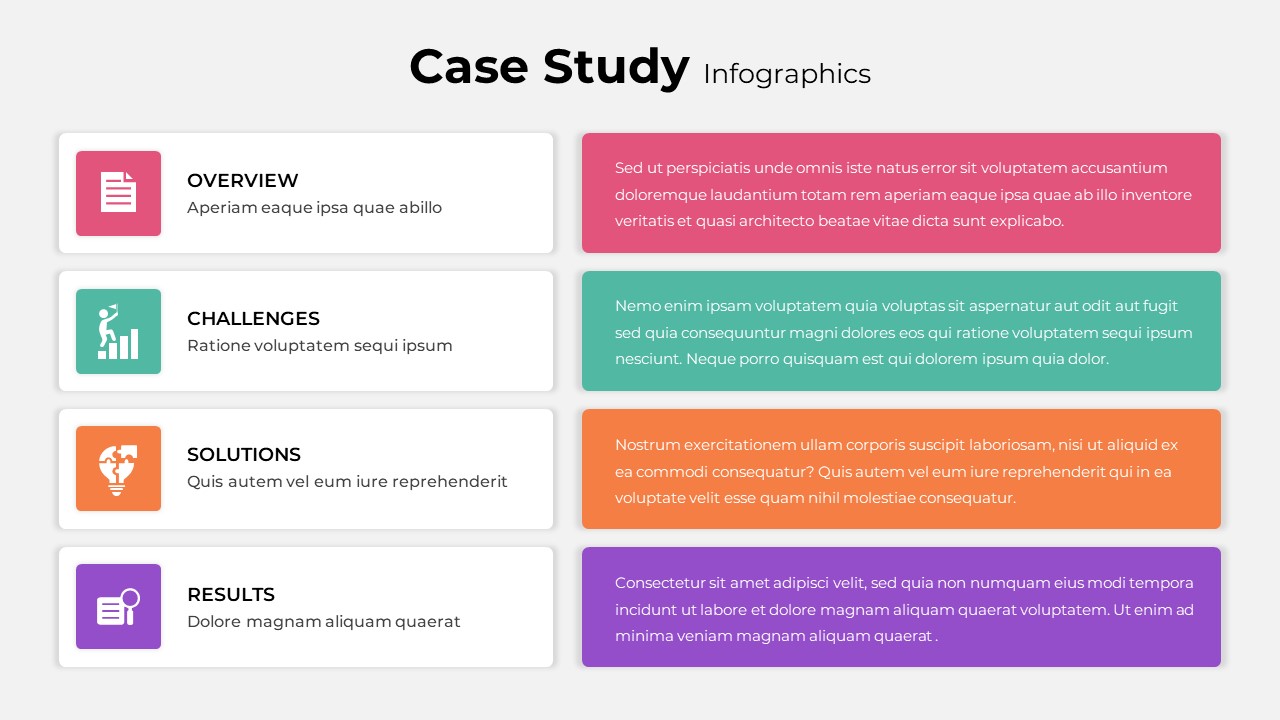
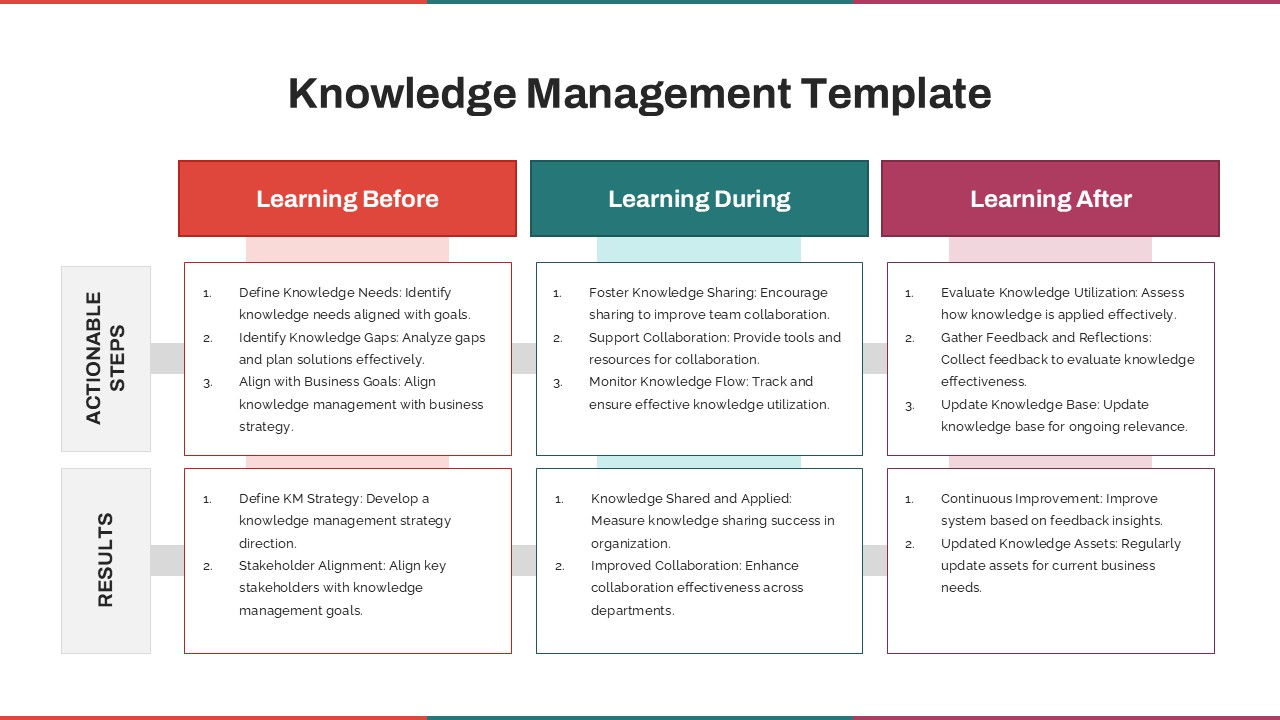
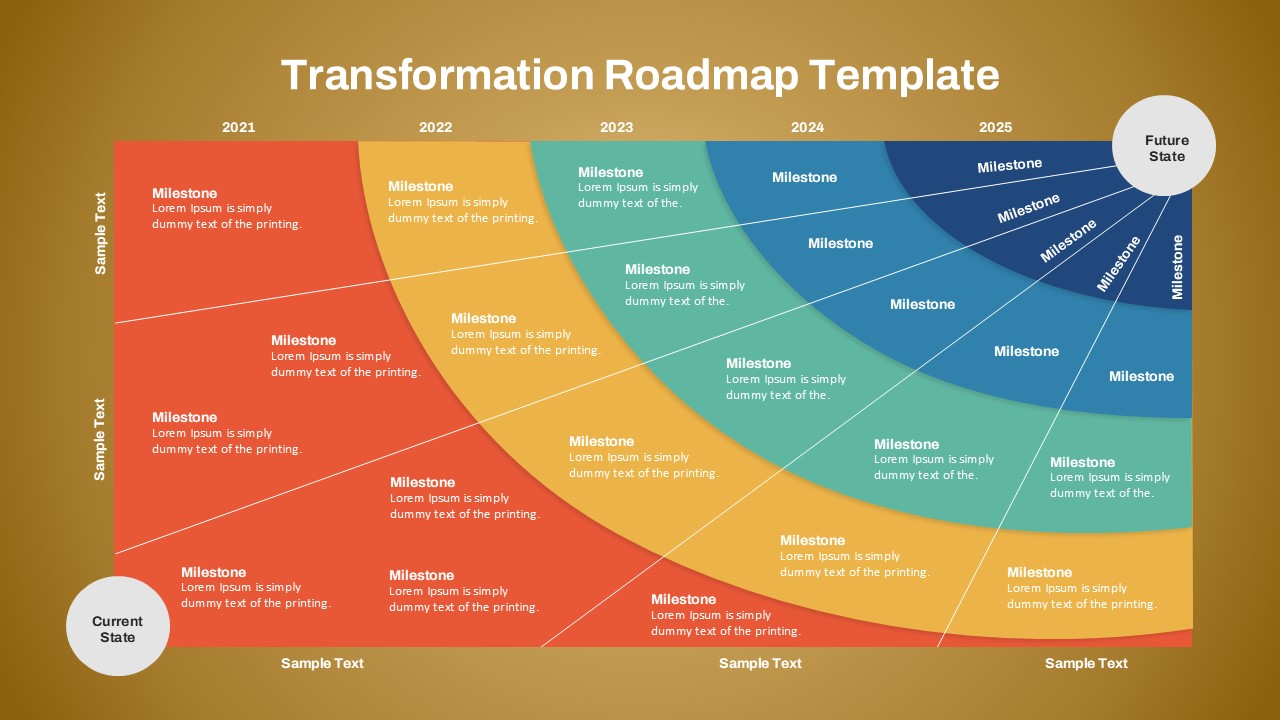
As one of the most effective roadmap slide examples for the healthcare and biotech sectors, this slide helps teams communicate progress, align cross-functional teams, and present to stakeholders with confidence. It’s ideal for funding pitches, internal project updates, and FDA readiness presentations. Additionally, the design is versatile enough to serve as a roadmap slide for other industries with milestone-heavy workflows.
Fully compatible with PowerPoint and Google Slides, this template is easy to edit and customize. Whether you’re preparing a high-stakes executive briefing or an investor report, this roadmap slide design offers clarity, professionalism, and impact.
See more
Features of this template
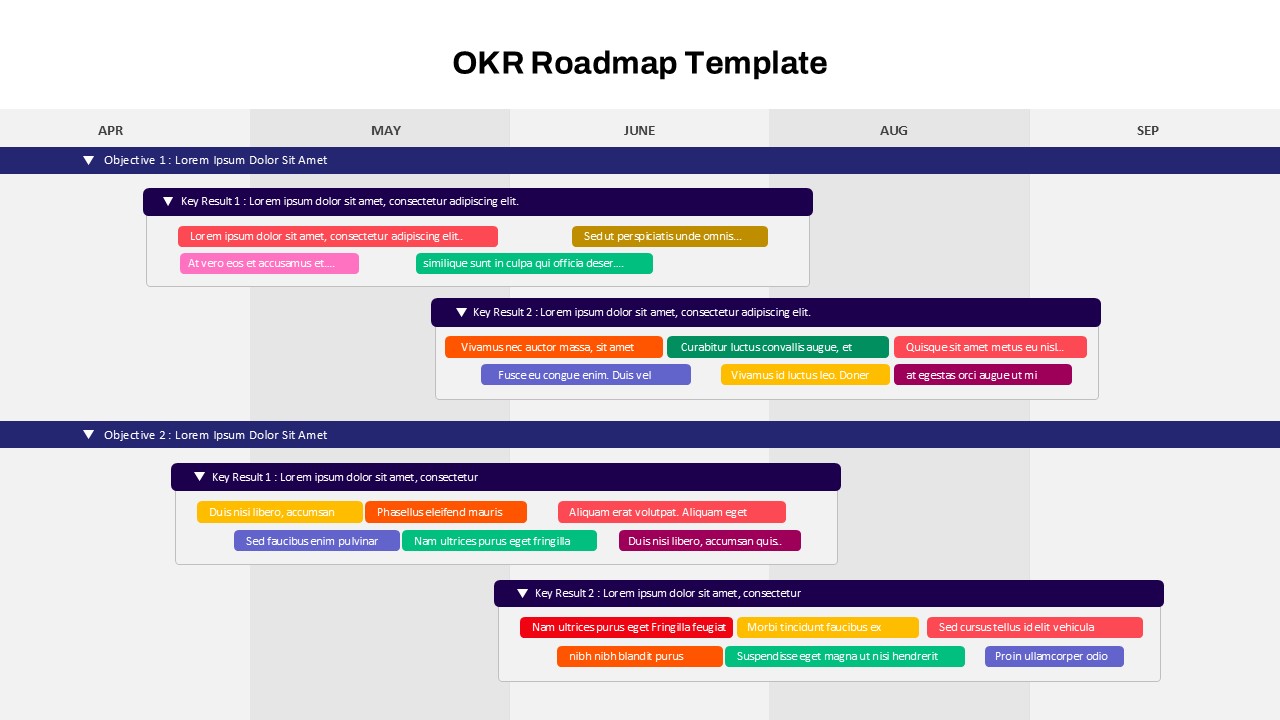
Other User Cases of the Template:
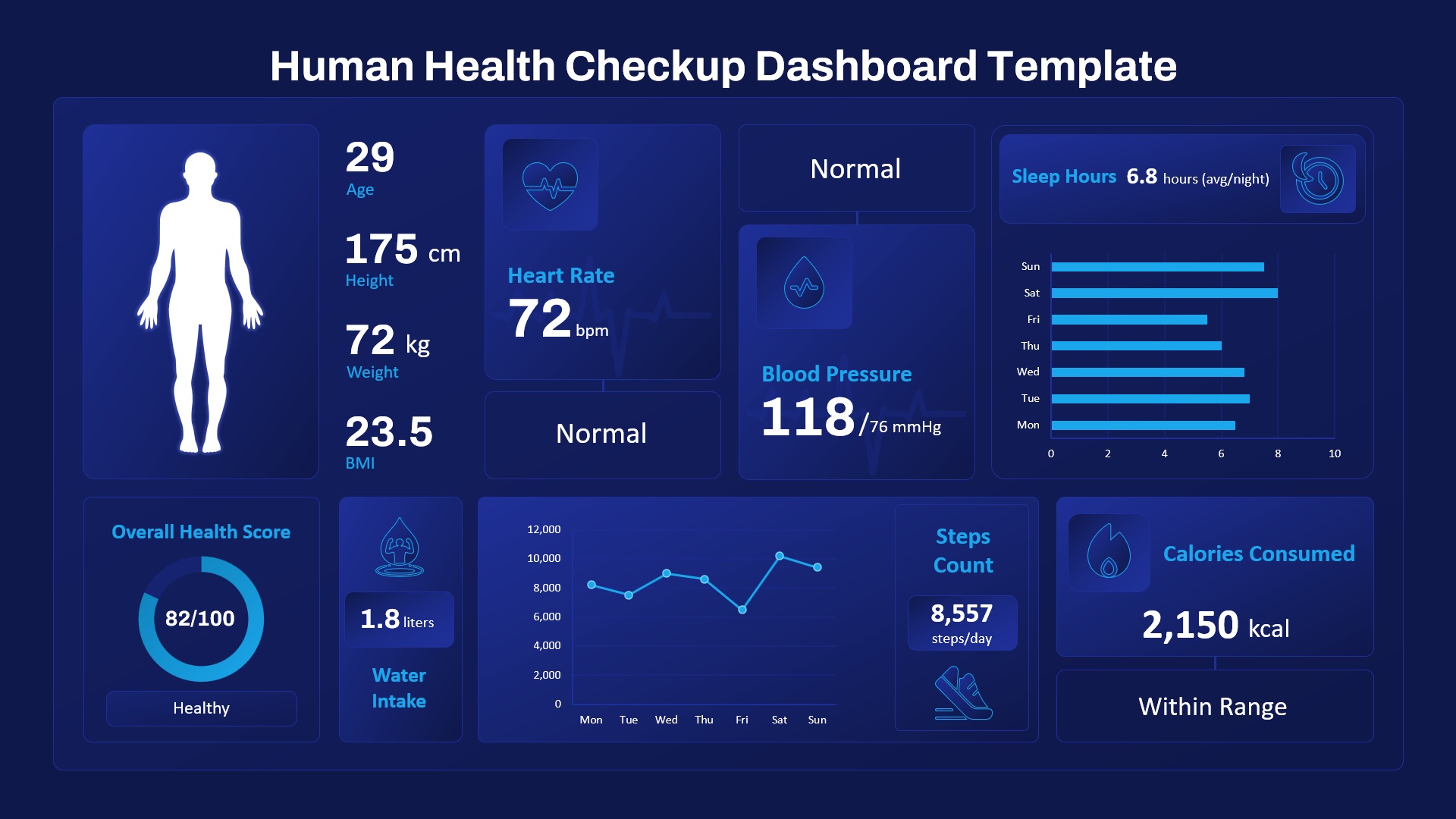
Pharmaceutical R&D planning, Biotech investor presentations, Clinical operations reviews, Regulatory submission timelines, Medical research grant proposals, Trial phase progress reports, Drug development timelines, CRO project tracking, FDA audit preparation, Medical study kick-off briefings